Mock test. 100 marks available.
Edexcel GCSE Chemistry Paper 1
Quiz Summary
0 of 74 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 74 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 74
1. Question
1 point(s)[question number = 01.1] Drag and drop to match the density with the state of matter
Sort elements
- Solid
- Gas
- Most dense state
- Least dense state
CorrectIncorrect -
Question 2 of 74
2. Question
6 point(s)[question number = 07.3] Match the change of state with the transition using the words below.
Freezing, sublimation, evaporation or boiling, condensation, melting, deposition
-
- Gas to liquid =
- Liquid to solid =
- Solid to liquid =
- Liquid to gas =
- Solid to gas =
- Gas to solid =
CorrectIncorrect -
-
Question 3 of 74
3. Question
3 point(s)[question number = 01.5] Drag and drop to match the description to the term
Sort elements
- Element
- Compound
- Mixture
- Contains only a particular type of atoms
- Contains more than one type of atoms, chemically bonded together
- Contains more than one type of atoms, but not chemically bonded together
CorrectIncorrect -
Question 4 of 74
4. Question
1 point(s)
The graph shows a heating curve for substance X (red) and substance Y (blue). Drag and drop to match the substance to the correct description.
Sort elements
- X (red)
- Y (blue)
- Pure
- Impure
CorrectIncorrect -
Question 5 of 74
5. Question
1 point(s)What is the chemical symbol for copper?
CorrectIncorrect -
Question 6 of 74
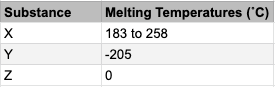
6. Question
1 point(s)
The table shows 3 substances and their melting points. What common substance, in particular, could substance Z be?
CorrectIncorrectHint
Which common, very important substance melts or freezes at 0 degrees C?
-
Question 7 of 74
7. Question
3 point(s)[question number = 01.2] Drag and drop to match the charge with the correct particle
Sort elements
- Protons
- Electrons
- Neutrons
- +1
- -1
- 0
CorrectIncorrect -
Question 8 of 74
8. Question
1 point(s)Drag and drop to match the description to the correct number
Sort elements
- Atomic number
- Mass number
- Number of protons
- Number of protons plus neutrons
CorrectIncorrect -
Question 9 of 74
9. Question
1 point(s)True or false. All atoms of the same element have the same atomic number.
CorrectIncorrect -
Question 10 of 74
10. Question
1 point(s)Which of the following things were hypothesised by John Dalton in 1805?
CorrectIncorrect -
Question 11 of 74
11. Question
1 point(s)Atomic number represents the number of protons in the nucleus of an atom. This means that elements further down the table have:
CorrectIncorrect -
Question 12 of 74
12. Question
1 point(s)In the modern periodic table, what are rows and columns called?
CorrectIncorrect -
Question 13 of 74
13. Question
1 point(s)Mendeleev did not know about isotopes, this caused his periodic table to have pair reversals where elements with a higher atomic weight were placed before elements with a lower atomic weight. Which subatomic particles are found in different numbers in isotopes?
CorrectIncorrect -
Question 14 of 74
14. Question
1 point(s)What is the electronic configuration of boron?
CorrectIncorrect -
Question 15 of 74
15. Question
1 point(s)Filtration and crystallisation are used to separate which types of materials?
CorrectIncorrect -
Question 16 of 74
16. Question
1 point(s)When a mixture of rocks, salt and water is filtered, what will be the residue?
CorrectIncorrect -
Question 17 of 74
17. Question
1 point(s)Fractional distillation is used to separate which types of materials?
CorrectIncorrect -
Question 18 of 74
18. Question
8 point(s)Use some of the following words to complete the statements below. You may use each word more than once.
Diamond, graphite, tetrahedral, delocalised, intermolecular, lattice, covalent, ionic
-
Complete the following statements:
- Graphite and diamond have high melting points because many strong bonds need to be broken
- does not conduct electricity because it has no electrons
- does conduct electricity because it has only three covalent bonds, leaving one electron from each atom.
- is soft because it is arranged in sheets with weak forces holding them together. These can easily slide over each other
- is hard because the carbon atoms are covalently bonded to 4 other carbon atoms, in a arrangement
CorrectIncorrect -
-
Question 19 of 74
19. Question
1 point(s)How do sodium and chlorine atoms bond?
CorrectIncorrect -
Question 20 of 74
20. Question
1 point(s)How many sulfur atoms will react with two lithium atoms to form an ionic compound?
CorrectIncorrect -
Question 21 of 74
21. Question
1 point(s)What do group one atoms do when they bond ionically with group 7 atoms?
CorrectIncorrect -
Question 22 of 74
22. Question
1 point(s)Which of these best describes metallic bonding?
CorrectIncorrect -
Question 23 of 74
23. Question
1 point(s)Why is magnesium a better electrical conductor than sodium?
CorrectIncorrect -
Question 24 of 74
24. Question
1 point(s)Halogens react with metals to form metal halides. Which kind of bond holds them together?
CorrectIncorrect -
Question 25 of 74
25. Question
1 point(s)A soluble base is called?
CorrectIncorrect -
Question 26 of 74
26. Question
1 point(s)What do acids produce when they dissolve in water?
CorrectIncorrect -
Question 27 of 74
27. Question
1 point(s)Can strong and weak acids have the same pH?
CorrectIncorrect -
Question 28 of 74
28. Question
6 point(s)Match the formula to the compound
Sodium hydroxide, hydrochloric acid, potassium hydroxide, nitric acid, calcium hydroxide, sulfuric acid
-
H₂SO₄ =
NaOH =
HCl =
KOH =
Ca(OH)₂ =
HNO₃ =
CorrectIncorrect -
-
Question 29 of 74
29. Question
1 point(s)Solutions of less than pH 7 are?
CorrectIncorrect -
Question 30 of 74
30. Question
1 point(s)Strong acids do what in water?
CorrectIncorrect -
Question 31 of 74
31. Question
1 point(s)Calculate the empirical formula of a compound that contains 69.94g of Fe (Aᵣ – 55.85) and 30.06g of O (Aᵣ – 12.00).
CorrectIncorrect -
Question 32 of 74
32. Question
1 point(s)Determine the empirical formula for Cyclopentane – C₅H₁₀?
CorrectIncorrect -
Question 33 of 74
33. Question
3 point(s)Closed, non-enclosed, neutralisation, precipitation, equal, non-equal
-
Complete the statement describing a chemical reaction:
Potassium iodide solution and lead nitrate solution react in a closed flask to form a yellow precipitate of lead iodide and a colourless solution of potassium nitrate. This is a reaction and since it occurs in a closed flask it is an example of a system. The mass of the reactants and products will be .
CorrectIncorrect -
-
Question 34 of 74
34. Question
1 point(s)Complete the following half equation for a reaction the cathode during electrolysis of molten zinc chloride. Zn²⁺ + ….. → Zn
CorrectIncorrect -
Question 35 of 74
35. Question
1 point(s)Complete the following half equation for a reaction the cathode during electrolysis of molten lead bromide. Pb²⁺ + 2e → …..
CorrectIncorrect -
Question 36 of 74
36. Question
1 point(s)During electrolysis which electrode do anions and cations migrate to?
CorrectIncorrect -
Question 37 of 74
37. Question
1 point(s)Place the metals in the correct order – from highest to lowest reactivity
- Iron
- Potassium
- Gold
- Magnesium
View Answers:
CorrectIncorrect -
-
Question 38 of 74
38. Question
1 point(s)Which of the following is the correct description of the reactivity of metals?
CorrectIncorrect -
Question 39 of 74
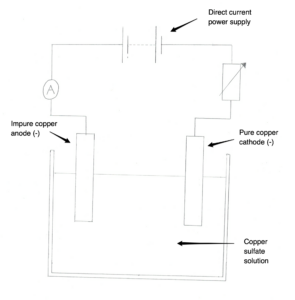
39. Question
1 point(s)Copper can be purified using electrolysis of copper sulfate solution. For this process, we use the setup in the image below. Complete the half equation for the reaction at the anode. Cu → ….. + …..
 CorrectIncorrect
CorrectIncorrect -
Question 40 of 74
40. Question
1 point(s)Which of the following is a typical property of transition metals?
CorrectIncorrect -
Question 41 of 74
41. Question
1 point(s)Which process results in the corrosion of metals?
CorrectIncorrect -
Question 42 of 74
42. Question
1 point(s)Select all methods that prevent rusting of iron.
CorrectIncorrect -
Question 43 of 74
43. Question
1 point(s)Use some of the following answers to fill in the blanks:
Appearance, hardness, weight, conductivity-
Electroplating is used to improve the and resistance to corrosion of metal objects.
CorrectIncorrect -
-
Question 44 of 74
44. Question
1 point(s)Why does converting pure metals into alloys often increase the strength of the product?
CorrectIncorrect -
Question 45 of 74
45. Question
1 point(s)Iron is alloyed with other metals to produce alloy steels in order to:
CorrectIncorrect -
Question 46 of 74
46. Question
1 point(s)Match each metal or alloy to its typical use.
Sort elements
- Aircraft manufacturing
- Jewellery
- Aluminium
- Gold
CorrectIncorrect -
Question 47 of 74
47. Question
1 point(s)Which piece of apparatus is used to accurately measure 25.0 cm³ of alkali in a titration?
CorrectIncorrect -
Question 48 of 74
48. Question
1 point(s)Calculate the percentage yield if the theoretical yield is 10 grams and the actual yield is 8 grams.
CorrectIncorrect -
Question 49 of 74
49. Question
1 point(s)Select all reasons why the actual yield of a reaction is less than the theoretical yield.
CorrectIncorrect -
Question 50 of 74
50. Question
1 point(s)The atom economy of a reaction is:
CorrectIncorrect -
Question 51 of 74
51. Question
1 point(s)At room temperature and pressure, one mole of any gas occupies a volume of:
CorrectIncorrect -
Question 52 of 74
52. Question
1 point(s)The Haber process is a reversible reaction between nitrogen and hydrogen to form ____.
CorrectIncorrect -
Question 53 of 74
53. Question
1 point(s)Select all factors that affect the rate of attainment of equilibrium.
CorrectIncorrect -
Question 54 of 74
54. Question
1 point(s)Fertilisers often contain compounds of which three elements?
CorrectIncorrect -
Question 55 of 74
55. Question
1 point(s)Ammonia reacts with nitric acid to produce which salt used as a fertiliser?
CorrectIncorrect -
Question 56 of 74
56. Question
1 point(s)In industrial production, ammonium sulfate is produced on a larger scale and involves:
CorrectIncorrect -
Question 57 of 74
57. Question
1 point(s)A chemical cell produces a voltage until:
CorrectIncorrect -
Question 58 of 74
58. Question
1 point(s)In a hydrogen–oxygen fuel cell, hydrogen and oxygen react to produce:
CorrectIncorrect -
Question 59 of 74
59. Question
1 point(s)Select all strengths of fuel cells.
CorrectIncorrect -
Question 60 of 74
60. Question
1 point(s)Sacrificial protection involves:
CorrectIncorrect -
Question 61 of 74
61. Question
1 point(s)Electroplating is a process that involves:
CorrectIncorrect -
Question 62 of 74
62. Question
2 point(s)Use some of the following answers to fill in the blanks:
Layers, slide, grains, melt-
In alloys, different sized atoms make it harder for to over each other.
CorrectIncorrect -
-
Question 63 of 74
63. Question
1 point(s)Match each metal to its typical property.
Sort elements
- Lightweight and strong
- Excellent electrical conductivity
- Magnalium
- Copper
CorrectIncorrect -
Question 64 of 74
64. Question
1 point(s)What is the volume of 2 moles of gas at room temperature and pressure?
CorrectIncorrect -
Question 65 of 74
65. Question
1 point(s)According to Avogadro’s law, equal volumes of gases at the same temperature and pressure contain:
CorrectIncorrect -
Question 66 of 74
66. Question
1 point(s)Select all factors considered when choosing a reaction pathway to produce a specified product.
CorrectIncorrect -
Question 67 of 74
67. Question
1 point(s)Which factor is NOT considered when choosing conditions for industrial reactions?
CorrectIncorrect -
Question 68 of 74
68. Question
1 point(s)In the Haber process, conditions are chosen to produce an acceptable yield in an acceptable time by controlling:
CorrectIncorrect -
Question 69 of 74
69. Question
2 point(s)Use some of the following answers to fill in the blanks:
Oxygen, water, rust, hydrogen-
The rusting of iron can be prevented by excluding and .
CorrectIncorrect -
-
Question 70 of 74
70. Question
1 point(s)Covalent compounds typically form between which two types of material?
CorrectIncorrect -
Question 71 of 74
71. Question
1 point(s)How many atoms of potassium (group 1) are needed to bond ionically with oxygen (group 6)?
CorrectIncorrect -
Question 72 of 74
72. Question
2 point(s)Metals and non-metals often have opposite properties. Drag and drop to match the typical property with the correct type of material.
Sort elements
- Metals
- Non-metals
- Good conductors of electricity
- Good insulators of electricity
- Shiny
- Dull in colour
- Metals
- Non-metals
CorrectIncorrect -
Question 73 of 74
73. Question
1 point(s)Most ionic bonds are formed between which groups?
CorrectIncorrect -
Question 74 of 74
74. Question
1 point(s)Oxygen is in group 6 and fluorine is in group 7. How many fluorine atoms bond with one oxygen atom?
CorrectIncorrect
Is this higher or foundation?
This is a combined paper for higher and foundation students. You can achieve grades 1 – 9 on this paper, so it is suitable for all. Grades are moderated against the average result to give the most accurate indication of your performance. You can look at – How is this paper marked? for more information.
How is this paper marked?
This paper is automatically marked to determine which questions were answered correctly.
Your grade is determined using a Z-Score moderation system. Your GCSE exams are also moderated comparably so that the difficulty of papers is taken into account.
Roughly, this works by calculating your overall percentage and comparing it to the average percentage and the standard deviation. This means that for harder papers you need fewer points to get the same grade as you would for an easier paper.
As more students attempt the paper, the average score and standard deviation more accurately represent the difficulty of the paper and the grades become more accurate.
Making these papers and the marking system took considerable effort so if you found them helpful for your revision, please show your appreciation by rating the page.
Edexcel Chemistry Paper 1
In this paper, knowledge of the various states of matter as well as the ways to separate materials from each other. This includes an introduction to the concepts of elements, compounds and mixtures. The overarching goal of this paper is for students to achieve a level of chemical literacy that will serve them throughout life and during A-level studies.
In SC3, students are tested on the structure of atoms as well as what makes an element unique and also the isotopes that exist in nature. SC4 links SC3 with an understanding of the Periodic Table and electronic configurations. SC5, SC6 and SC8 ask students about how various chemicals bond and how this is related to their properties and characteristics. SC8 quizzes students on their knowledge of acids and alkalis including the basics and more in-depth topics like neutralisation. SC9 tests students’ ability to carry out accurate calculations involving masses. SC10, SC11, SC12 and SC13 are an extension of the materials science section whereby students are asked to explain the process of electrolysis, how metals are obtained and used and also how reversible reactions take place. The final topics, SC14, SC15 and SC16 aim to test for quantitative analysis skills and understanding of dynamic equilibria and how fuel cells work.
If you would like to try another Edexcel Quiz why not try out the first biology paper, Edexcel GCSE Biology Paper 1.
Related Quizzes
Which exam board are you studying?