Do you know the difference between an element, a compound and a mixture?
Elements, Compounds and Mixtures Quiz
Good Luck!
Quiz Summary
0 of 8 Questions completed
Questions:
Information
You have already completed this quiz. You cannot start it again.
Quiz is loading…
You must sign in or sign up to take this quiz.
You must first complete the following:
Results
Quiz complete. Results are being recorded.
Results
0 of 8 Questions answered correctly
Your Time:
Time has elapsed.
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average Score |
|
| Your Score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 8Question 1
Which of the following describe the composition of a pure substance?
CorrectIncorrect -
Question 2 of 8Question 2
Which types of atoms can be found in a pure piece of gold?
CorrectIncorrect -
Question 3 of 8Question 3
Drag and drop to match the description to the term
- Element
- Compound
- Mixture
-
Contains only a particular type of atoms
-
Contains more than one type of atoms, chemically bonded together
-
Contains more than one type of atoms, but not chemically bonded together
CorrectIncorrect -
Question 4 of 8Question 4
Drag and drop to match the substance to the correct type
- Mixture
- Compound
- Element
-
Air
-
Carbon dioxide
-
Pure gold
CorrectIncorrect -
Question 5 of 8Question 5
-
Fill in the blank. A is a mixture made of dissolved substances in a liquid.
CorrectIncorrectUse one of the following words:
solution
solute
solvent
solent
-
-
Question 6 of 8Question 6

The graph shows a heating curve for substance X (red) and substance Y (blue). Drag and drop to match the substance to the correct description.
- X (red)
- Y (blue)
-
Pure
-
Impure
CorrectIncorrect -
Question 7 of 8Question 7

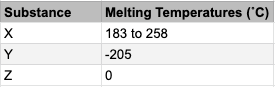
The table shows 3 substances and their melting points. Drag and drop to match the substance to the correct type.
- Mixture
- Element or compound
-
X
-
Y
CorrectIncorrect -
Question 8 of 8Question 8

The table shows 3 substances and their melting points. What common substance, in particular, could substance Z be?
CorrectIncorrectWhich common, very important substance melts or freezes at 0 degrees C?
Read our post on elements, compounds and mixtures to learn about their differences and why they are important in chemistry.
Which exam board are you studying?