Mock test. 1 hour and 10 minutes. 60 marks available.
Edexcel Combined Science Paper 3: Chemistry 1
Is this higher or foundation?
This is a combined paper for higher and foundation students. You can achieve grades 1 – 9 on this paper, so it is suitable for all. Grades are moderated against the average result to give the most accurate indication of your performance. You can look at – How is this paper marked? for more information.
How is this paper marked?
This paper is automatically marked to determine which questions were answered correctly.
Your grade is determined using a Z-Score moderation system. Your GCSE exams are also moderated comparably so that the difficulty of papers is taken into account.
Roughly, this works by calculating your overall percentage and comparing it to the average percentage and the standard deviation. This means that for harder papers you need fewer points to get the same grade as you would for an easier paper.
As more students attempt the paper, the average score and standard deviation more accurately represent the difficulty of the paper and the grades become more accurate.
Making these papers and the marking system took considerable effort so if you found them helpful for your revision, please show your appreciation by rating the page.
What does Edexcel Combined Science Paper 3: Chemistry 1 cover?
This paper covers the absolute basics of chemistry which are required to move on with the course. Students are tested on their understanding of states of matter which are fundamentally important to understanding the concepts of acids and alkalis. This first chemistry paper aims to test a student’s knowledge of topics that will help them to make sense of the world around them as well as understand the risks associated with certain materials. Additionally, the test will prepare them for A-level study or can be useful for a number of job roles by using the tools of logic in the workplace.
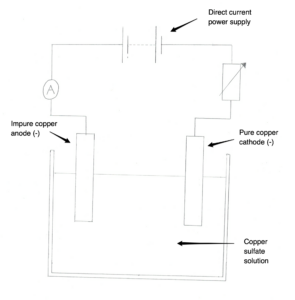
In this paper, the concepts of atomic structure which again are fundamental to later topics are addressed in CC1. CC2 continues on from CC1 with the periodic table and elements, along with concepts like electronic configurations and the basics of interactions between elements. CC5 tests students’ knowledge of ionic bonding which presupposes an understanding of the atomic structure and subatomic particles. CC6 adds in covalent bonding before CC7 addresses the rest of the bonding models which attempt to explain the properties of the materials around us. Acids and alkalis are next in CC8 where the students are expected to understand reactions between different materials, and the products and annotate or write balanced chemical equations including state symbols. Half and balanced ionic equations are assessed depending on the level. CC9 tests knowledge of masses, moles and empirical formulae. CC10 asks about electrolytic processes which link to CC11, obtaining and using metals and finally, CC12, reversible reactions and equilibria.
If you feel like you’re ready, move on to the next mock test – Edexcel Combined Science Paper 4: Chemistry 2.