Do you know how things bond?
Topic Overview - Bonding Models
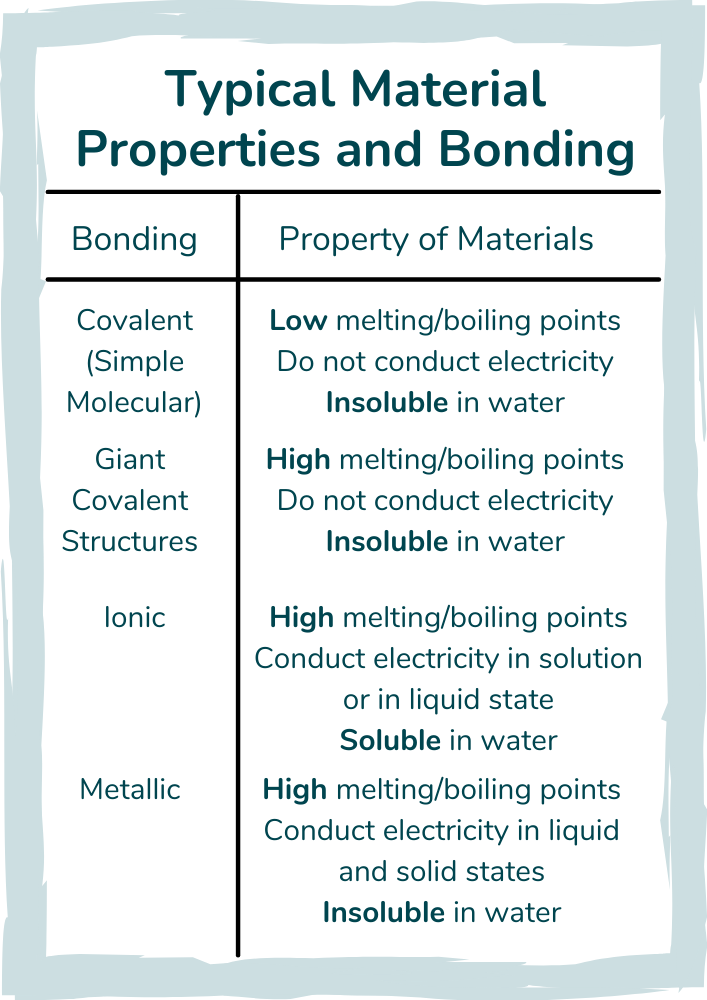
The bonding models quiz is an overview of the way that materials bond to make compounds, including ionic, covalent and metallic bonding.
If you would like to learn more about the basics of how these types of bonding work, then check out our atomic structure quiz and also our elements, compounds and mixtures quiz.