Mock test. 100 marks available.
Edexcel GCSE Chemistry Paper 2
Quiz Summary
0 of 75 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 75 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 75
1. Question
1 point(s)Which subatomic particles surround the nucleus in specific energy levels also known as shells?
CorrectIncorrect -
Question 2 of 75
2. Question
1 point(s)Which of the following is the correct maximum number of electrons in the first three shells?
CorrectIncorrect -
Question 3 of 75
3. Question
1 point(s)Calculate the mass of fluorine needed to make 100g of magnesium fluoride. Consult the periodic table for all of the information you will need. Answer to one decimal place.
The balanced chemical equation for this reaction is:
F₂ + Mg → MgF₂
-
g
CorrectIncorrect -
-
Question 4 of 75
4. Question
1 point(s)Why can we use the periodic table to find out if an element is one of the following three: Alkali metals, noble gases or halogens?
CorrectIncorrect -
Question 5 of 75
5. Question
2 point(s)Which of the following are properties of alkali metals?
CorrectIncorrect -
Question 6 of 75
6. Question
4 point(s)Use some of the following words to fill in the blanks.
Lower, higher, increase, decrease, chlorine, iodine
-
Complete the statement:
As you go down group 7, density, melting points and boiling points .
That means that fluorine will have a boiling point than chlorine.
So, bromine is denser than and chlorine has a boiling point than iodine.
CorrectIncorrect -
-
Question 7 of 75
7. Question
1 point(s)Chemical reactions require a high enough collision energy for reactions to take place. What do we call the minimum amount of energy needed for a reaction?
CorrectIncorrect -
Question 8 of 75
8. Question
1 point(s)In an experiment to measure the rate of photosynthesis of pondweed in a test tube, the number of bubbles (oxygen) produced per minute is used to determine the rate of reaction. What is this experiment actually measuring?
CorrectIncorrect -
Question 9 of 75
9. Question
1 point(s)Drag and drop to match the outcome with the type of reaction
Sort elements
- Exothermic
- Endothermic
- If there is no energy input – reactions continue until reactants run out
- If there is no energy input – reactions stop even if there are reactants left
CorrectIncorrect -
Question 10 of 75
10. Question
1 point(s)Temperature affects the rate of reaction. Which of the following is the correct description of what happens when temperature changes?
CorrectIncorrect -
Question 11 of 75
11. Question
1 point(s)Which of these describes the term “rate of reaction”?
CorrectIncorrect -
Question 12 of 75
12. Question
1 point(s)Why is a spark often needed to start a combustion reaction?
CorrectIncorrect -
Question 13 of 75
13. Question
6 point(s)[question number = 01.4] Use some of the following words to complete the statements below. You may use each word more than once.
Increase, increases, decrease, decreases, lower, higher
-
Complete the following statements about the trends in the properties of noble gases as you go down the group:
- Melting points . e.g. helium has a melting point than neon
- Boiling points . e.g. neon has a melting point than argon
- Density . e.g. krypton has a density than argon
CorrectIncorrect -
-
Question 14 of 75
14. Question
1 point(s)
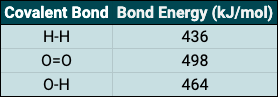
A bond energy is the energy needed to break one mole of a particular covalent bond. Calculate the overall bond energies of the following reaction using the table provided. 2(H-H) + O=O → 2(H-O-H)
CorrectIncorrect -
Question 15 of 75
15. Question
2 point(s)Ectothermic, mesothermic, exothermic, endothermic
-
Complete the sentence: Breaking bonds is and making bonds is
Correct 2 / 2 PointsIncorrect / 2 PointsHint
-
-
Question 16 of 75
16. Question
1 point(s)Arrange the following steps in the correct order to describe how crude oil is separated into more useful fractions.
- Fuel oil leaves second, followed by diesel oil.
- Crude oil is heated until it evaporates and then is pumped into the bottom of a fractionating column.
- When hydrocarbons condense they drip into a tray and are removed in pipes.
- As the vapours rise they cool and different hydrocarbons condense due to their difference in boiling points.
- At the top of the column, some gases leave as they have still not condensed.
- Kerosene condenses and is removed.
- Petrol condenses and is removed.
- Bitumen leaves first since it has the lowest boiling point and the column is hotter at the bottom.
View Answers:
CorrectIncorrect -
-
Question 17 of 75
17. Question
1 point(s)[question number = 01.8] Hydrocarbons are compounds that contain which two elements only?
CorrectIncorrect -
Question 18 of 75
18. Question
1 point(s)Hydrocarbons with the longest carbon chains have the highest boiling points. Where are the fractions with the longest carbon chains removed in a fractionating column?
CorrectIncorrect -
Question 19 of 75
19. Question
1 point(s)Which gas that leads to acid rain is produced when hydrocarbons containing sulfur are burned?
CorrectIncorrect -
Question 20 of 75
20. Question
1 point(s)Which process do we use to separate crude oil into more useful and simpler mixtures?
CorrectIncorrect -
Question 21 of 75
21. Question
1 point(s)Alkanes and alkenes are both part of a series, what is the name we give to the series?
CorrectIncorrect -
Question 22 of 75
22. Question
1 point(s)Which of the following are effects of climate change?
CorrectIncorrect -
Question 23 of 75
23. Question
2 point(s)[question number = 04.3] Why do historical measurements by humans of global temperature have some uncertainty?
Correct 2 / 2 PointsIncorrect / 2 Points -
Question 24 of 75
24. Question
4 point(s)[question number = 04.1] Which steps can we take to reduce the impact of climate change on humanity or prevent further climate change?
Correct 4 / 4 PointsIncorrect / 4 Points -
Question 25 of 75
25. Question
1 point(s)Why may our attempts to mitigate the effects of climate change cause further damage to the environment?
CorrectIncorrect -
Question 26 of 75
26. Question
1 point(s)Which of the following examples matches the composition of Earth’s early atmosphere?
CorrectIncorrect -
Question 27 of 75
27. Question
4 point(s)Use some of the following words to fill in the blanks:
Oxygen, carbon dioxide, methane, water vapour, ultraviolet, infrared, radio
-
Energy from the sun in the form of visible waves hits the Earth’s surface and waves are emitted back towards the atmosphere.
Greenhouse gases such as , and absorb infrared radiation from the Earth and re-emit it back to the Earth’s surface, causing warming.
Correct 4 / 4 PointsIncorrect / 4 PointsHint
Use some of the following words:
infrared
carbon dioxide
water vapour
methane
oxygen
-
-
Question 28 of 75
28. Question
1 point(s)[question number = 03.4] What is the name of the force that holds together ions in ionic compounds?
CorrectIncorrect -
Question 29 of 75
29. Question
1 point(s)Which of the following are properties of ionic compounds?
CorrectIncorrect -
Question 30 of 75
30. Question
1 point(s)Would astatine displace chlorine in an aqueous ionic compound of sodium chloride?
CorrectIncorrect -
Question 31 of 75
31. Question
1 point(s)Why are some metals extracted through heating with carbon rather than through electrolysis?
CorrectIncorrect -
Question 32 of 75
32. Question
1 point(s)How many particles are there in a mole of a substance?
CorrectIncorrect -
Question 33 of 75
33. Question
1 point(s)Calculate the mass of 16 moles of hydrochloric acid.
Click the hint to see the formula for hydrochloric acid, you need to remember this as well as other common acids.
CorrectIncorrectHint
HCl is the formula for hydrochloric acid.
Whilst you’re here, here’s the formulae for other important acids you need to remember…
Nitric acid = HNO3
Sulfuric acid = H₂SO₄
-
Question 34 of 75
34. Question
3 point(s)[question number = 05.1] Calculate the number of molecules in 16g of oxygen O₂.
CorrectIncorrect -
Question 35 of 75
35. Question
1 point(s)Complete the following half equation for a reaction the cathode during electrolysis of molten lead bromide. Pb²⁺ + 2e → …..
CorrectIncorrect -
Question 36 of 75
36. Question
1 point(s)During electrolysis which electrode do anions and cations migrate to?
CorrectIncorrect -
Question 37 of 75
37. Question
2 point(s)Use some of the following words to fill in the blanks:
Loss, gain, anode, cathode-
Fill in the blanks: Reduction refers to the of electrons and it occurs at the
Correct 2 / 2 PointsIncorrect / 2 PointsHint
Use some of the following words:
anode
cathode
loss
gain
-
-
Question 38 of 75
38. Question
7 point(s)Use the following terms to fill in the blanks. Use each term more than once.
Phytoextraction, bioleaching, both
-
Match the advantages and disadvantages with the correct method of metal extraction:
- No harmful gases (e.g. sulfur dioxide) are produced –
- Is a very slow process –
- Does not require high temperatures –
- Toxic substances and sulfuric acid which are produced as a byproduct can damage the environment –
- Can be used to extract metals from contaminated soils –
- Is more expensive than mining some ores –
- Growing plants is dependent on weather and climate conditions –
CorrectIncorrect -
-
Question 39 of 75
39. Question
1 point(s)Why must the test for any ion be unique?
CorrectIncorrect -
Question 40 of 75
40. Question
1 point(s)Match each ion to its corresponding flame colour.
Sort elements
- Red
- Lilac
- Lithium ion, Li⁺
- Potassium ion, K⁺
CorrectIncorrect -
Question 41 of 75
41. Question
1 point(s)Which ion forms a blue precipitate when sodium hydroxide solution is added?
CorrectIncorrect -
Question 42 of 75
42. Question
1 point(s)Which gas turns damp red litmus paper blue?
CorrectIncorrect -
Question 43 of 75
43. Question
1 point(s)Which ion can be identified by adding dilute acid and observing effervescence due to carbon dioxide gas?
CorrectIncorrect -
Question 44 of 75
44. Question
1 point(s)Which ion forms a white precipitate when dilute hydrochloric acid and barium chloride solution are added?
CorrectIncorrect -
Question 45 of 75
45. Question
3 point(s)Match each halide ion to the colour of precipitate formed when dilute nitric acid and silver nitrate solution are added.
Sort elements
- White precipitate
- Yellow precipitate
- Chloride ion, Cl⁻
- Iodide ion, I⁻
CorrectIncorrect -
Question 46 of 75
46. Question
1 point(s)Which of the following is a benefit of using instrumental methods of analysis?
CorrectIncorrect -
Question 47 of 75
47. Question
1 point(s)What can flame photometry be used for?
CorrectIncorrect -
Question 48 of 75
48. Question
1 point(s)What is the molecular formula of butane?
CorrectIncorrect -
Question 49 of 75
49. Question
1 point(s)Why are alkanes considered saturated hydrocarbons?
CorrectIncorrect -
Question 50 of 75
50. Question
1 point(s)What is the general formula for alkenes?
CorrectIncorrect -
Question 51 of 75
51. Question
1 point(s)What functional group do alkenes contain that makes them unsaturated?
CorrectIncorrect -
Question 52 of 75
52. Question
1 point(s)What is the product when ethene reacts with bromine?
CorrectIncorrect -
Question 53 of 75
53. Question
1 point(s)How can bromine water be used to distinguish between alkanes and alkenes?
CorrectIncorrect -
Question 54 of 75
54. Question
1 point(s)What are the products of the complete combustion of alkanes and alkenes?
CorrectIncorrect -
Question 55 of 75
55. Question
1 point(s)What is a polymer?
CorrectIncorrect -
Question 56 of 75
56. Question
1 point(s)In addition polymerisation, how do ethene molecules form poly(ethene)?
CorrectIncorrect -
Question 57 of 75
57. Question
1 point(s)Match each monomer to the polymer it forms through addition polymerisation.
Sort elements
- Poly(propene)
- Poly(tetrafluoroethene) (PTFE)
- Propene
- Tetrafluoroethene
CorrectIncorrect -
Question 58 of 75
58. Question
1 point(s)Which monomer is used to make poly(propene)?
CorrectIncorrect -
Question 59 of 75
59. Question
1 point(s)Which property of PTFE makes it suitable for use in non-stick cookware?
CorrectIncorrect -
Question 60 of 75
60. Question
1 point(s)Why are polyesters classified as condensation polymers?
CorrectIncorrect -
Question 61 of 75
61. Question
1 point(s)Select all the problems associated with disposal of polymers:
CorrectIncorrect -
Question 62 of 75
62. Question
1 point(s)Which of the following is an advantage of recycling polymers?
CorrectIncorrect -
Question 63 of 75
63. Question
1 point(s)DNA is a natural polymer made from monomers called what?
CorrectIncorrect -
Question 64 of 75
64. Question
1 point(s)What is the functional group present in all alcohols?
CorrectIncorrect -
Question 65 of 75
65. Question
1 point(s)In the combustion of alcohols, how does the number of carbon atoms in the alcohol affect the energy released?
CorrectIncorrect -
Question 66 of 75
66. Question
1 point(s)What is formed when ethanol is oxidised?
CorrectIncorrect -
Question 67 of 75
67. Question
1 point(s)Which of the following best describes the size range of nanoparticles?
CorrectIncorrect -
Question 68 of 75
68. Question
1 point(s)Which of the following is a potential risk of using nanoparticles?
CorrectIncorrect -
Question 69 of 75
69. Question
3 point(s)Match each material to its typical property.
Sort elements
- Good electrical conductivity
- Low density and poor electrical conductivity
- Metals
- Polymers
CorrectIncorrect -
Question 70 of 75
70. Question
1 point(s)Which material is most suitable for making electrical wiring?
CorrectIncorrect -
Question 71 of 75
71. Question
1 point(s)Members of a homologous series have similar chemical properties because they have the same what?
CorrectIncorrect -
Question 72 of 75
72. Question
1 point(s)What is the functional group present in carboxylic acids?
CorrectIncorrect -
Question 73 of 75
73. Question
1 point(s)What product is formed when an alcohol is dehydrated?
CorrectIncorrect -
Question 74 of 75
74. Question
1 point(s)What is the molecular formula of ethanoic acid?
CorrectIncorrect -
Question 75 of 75
75. Question
2 point(s)Use some of the following answers to fill in the blanks:
Dilute nitric acid, dilute hydrochloric acid, barium chloride solution, silver nitrate solution-
To test for sulfate ions, add followed by .
CorrectIncorrect -
Is this higher or foundation?
This is a combined paper for higher and foundation students. You can achieve grades 1 – 9 on this paper, so it is suitable for all. Grades are moderated against the average result to give the most accurate indication of your performance. You can look at – How is this paper marked? for more information.
How is this paper marked?
This paper is automatically marked to determine which questions were answered correctly.
Your grade is determined using a Z-Score moderation system. Your GCSE exams are also moderated comparably so that the difficulty of papers is taken into account.
Roughly, this works by calculating your overall percentage and comparing it to the average percentage and the standard deviation. This means that for harder papers you need fewer points to get the same grade as you would for an easier paper.
As more students attempt the paper, the average score and standard deviation more accurately represent the difficulty of the paper and the grades become more accurate.
Making these papers and the marking system took considerable effort so if you found them helpful for your revision, please show your appreciation by rating the page.
Edexcel Chemistry Paper 2
This paper attempts to assess the understanding of GCSE students on a variety of chemistry topics that are covered in the second half of the course. As usual, the overarching goal is to prepare students for leaving school, whether that be to gain employment or to move on to further education. There are some common elements in this paper with paper 1, including atomic structure, the periodic table, ionic bonding, covalent bonding as well as types of substances and their properties. Additionally, both tests cover calculations involving masses.
The test includes coverage of some of the important groups in the periodic table such as groups 1, 7 and 0 as well as a look at halogens. Knowledge of rates of reactions is assessed in SC18 alongside heat energy changes in chemical reactions (SC19). SC20 covers the various fuels and combustion, whilst SC21 addresses Earth and atmospheric science, specifically looking at how the atmosphere changes over time and how humans are influencing climate change. Hydrocarbons, alcohols and carboxylic acids are looked at next in SC22 and SC23 respectively. SC24 qualifies students’ learning on polymers and how they are used along with their properties. SC25 tests for knowledge of tests for positive and negative ions. Finally, SC26 tests for understanding the bulk and surface properties of matter, choosing materials, composites and nanoparticles.
If you would like to try another Edexcel Quiz why not try out the first chemistry paper, Edexcel GCSE Chemistry Paper 1.
Which exam board are you studying?