Ionic bonding is where metal and non-metal atoms form bonds held together with electrostatic attraction. Metal atoms give the non-metal atoms electrons and they both form a full outer shell. The metal forms a positive ion and the non-metal, a negative ion. Opposite charges attract, forming ionic bonds.
Ionic Bonding Explained
What is Ionic Bonding and How Does it Work?
Ionic bonding is a particular way in which some elements bond to form molecules or compounds. Typically this happens between metals and non-metals.
At the GCSE level, you will mainly need to work with groups 1, 2, 6 and 7.
In order to explain how this works, we will investigate the bonding of the compound sodium chloride, most commonly known as table salt.

Sodium Atomic Structure
How many electrons does sodium have? This question is a good place to start on this short journey, but first, we must ask the question “how many protons and neutrons do sodium atoms have?”. Sodium, which has the atomic symbol Na, has an atomic number of 11 and a mass number of 23. This means that sodium atoms have 11 protons and 12 neutrons. Atoms are neutral and have no charge, whilst protons have a charge of +1 and electrons -1. These opposite charges mean that the number of electrons must be equal to the number of protons, so sodium atoms have 11 electrons!
Sodium Electronic Configuration
Now that we know the number of protons, neutrons and electrons in a sodium atom we can consider the electronic configuration of sodium atoms. For reasons best explained in another article, the number of electrons that orbit in shells follows the pattern of 2,8,8, meaning that the first electron shell holds two electrons, the second shell holds 8 electrons and the third shell holds only one electron.
A quick trick to figure out how many electrons are in the outer shell is to look up the group number of an element in the periodic table. The group number, which in the case of sodium is one, is equal to the number of electrons in the outer shell.
Bonding in Sodium Chloride
So how does sodium chloride bond? We have so far explored half of the ingredients for a molecule of NaCl (sodium chloride), now we will quickly go over the chlorine half.
Chlorine has 17 protons and therefore 17 electrons and since it is in group 7, we know that there will be one electron in its outer shell. So the electronic configuration of chlorine is 2,8,7. Now you might notice that full outer shells beyond the first shell have 8 electrons, this is an important point and having a full outer shell brings stability to compounds.
Elements in groups 1,2,6 and 7 are notoriously reactive because they have to only gain or lose a few electrons in order to become stable. After all, atoms want to have full outer shells and their electrons will move between atoms or become shared between atoms to make this happen.
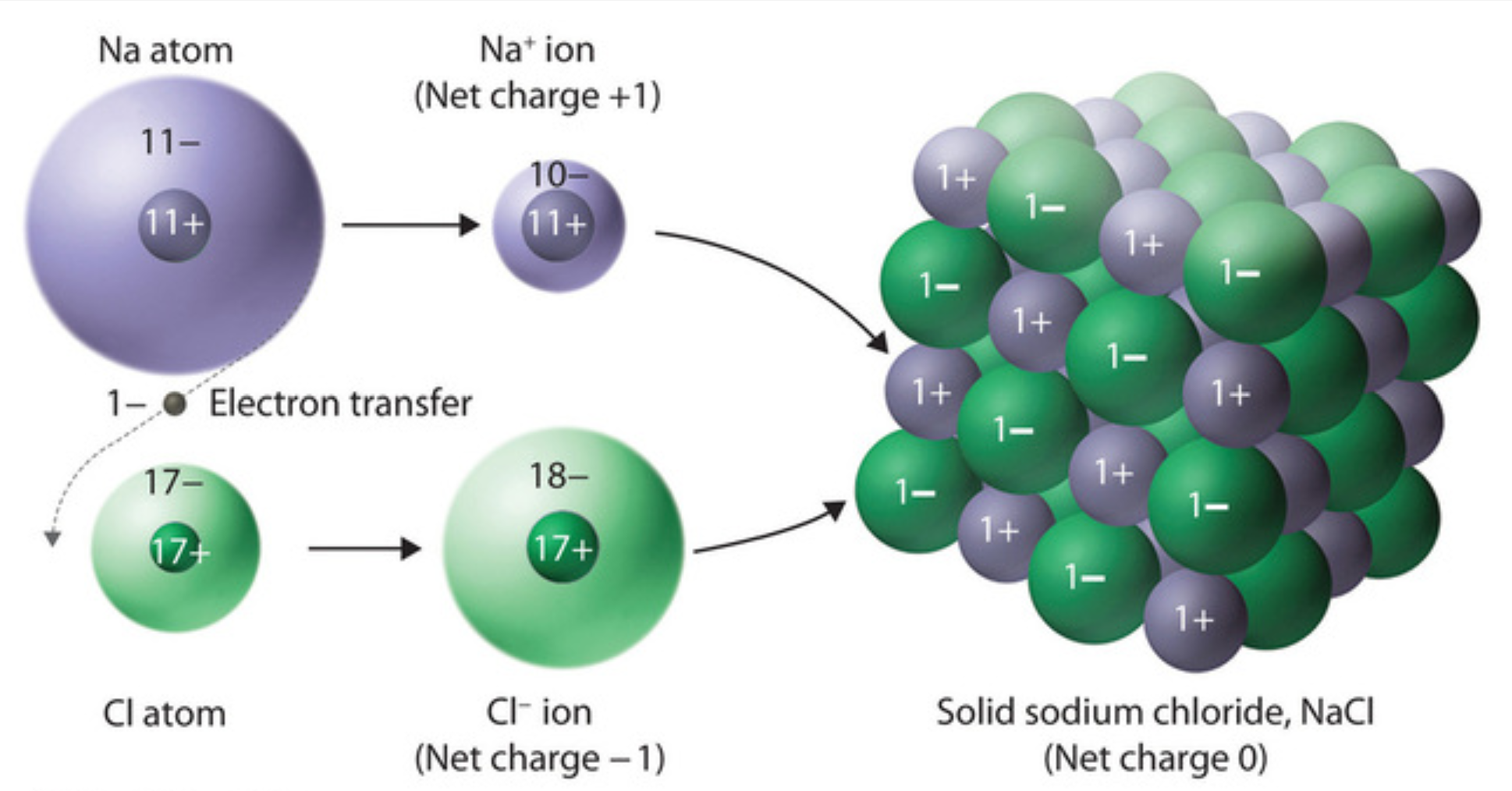
Sodium chloride bonds ionically, this means that ions are formed when an electron from the sodium, the spare electron, jumps to the outermost electron shell of chlorine. Once this happens the electron shells of each atom are full and stability is achieved.
However, the -1 charge of electrons means that the once neutral atoms have now become ions, the sodium with a +1 charge and the chlorine with a -1 charge. These now charged particles are attracted to each other via electrostatic forces, a strong bond that influences their properties. NaCl forms a lattice structure that makes the crystals we are familiar with.
Properties of Ionic Compounds
Because of the strong electrostatic forces holding ions together in ionic compounds, they are difficult to split apart and have high melting and boiling temperatures. Additionally, they are excellent conductors of electricity when molten or dissolved because the ions, when split apart from each other are able to carry a charge. Generally, ionic compounds are soluble.
If you want to find out more about ionic bonding, covalent bonding and more, reach out to Discover Tutoring and see how we can help you.
Why is Chlorine More Reactive than Bromine?
The reactivity of the halogens (group 7 elements) is determined by the number of electron shells and therefore the atomic radius of the elements.
Each time you go down a period (row) on the periodic table, the element has one more electron shell. Chlorine is one period higher than bromine and so has one less electron shell. Since the outermost electron shell is closer to the positive nucleus when there are fewer shells, the electrostatic attraction will be stronger been the nucleus and the outermost shell. This stronger force of attraction allows chlorine to acquire new electrons (usually from group 1 and 2 metals) and ionically bond more easily than bromine, making it more reactive.
This is actually the opposite to group 1 elements which are more reactive as you go down the group. This is because as the atomic radius increases, the ability of the nucleus to hold on to its outermost electrons is reduced, making them more likely to form positive ions.
Elements also bond through two other bonding models – covalent bonding and metallic bonding.
For more fundamental chemistry knowledge, check out the atomic structure quiz and our page on elements, compounds and mixtures.
