Covalent bonding occurs between non-metal elements such as hydrogen and oxygen. One or more electrons in the outermost shells are shared between atoms to complete the shells. Opposite charges attract and so the atoms are held together by their mutual attraction to the shared electrons since the overall charge of electrons is -1 and an atomic nucleus is +1.
Covalent Bonding Explained
We will use oxygen molecule covalent bonding as an example to help understand the concepts
Oxygen structure and the periodic table
Oxygen is a non-metal element in group 6 of the periodic table (sometimes written as group 16). This group number tells us that there are 6 electrons in the outermost electron shell. Also, remember that atoms are electrically neutral because the number of protons (+1 charge) and electrons (-1 charge) are equal. Learn more about atomic structure.
Remember, when two non-metals come together they form covalent bonds. Two metals would form a metallic bond and a metal and a non-metal would form an ionic bond.
Oxygen electronic structure explained with a dot and cross diagram
Oxygen has 6 electrons in its outermost shell. That means it needs two electrons to gain a complete outer shell.
Two oxygen atoms come together and form a double covalent bond by sharing their two electrons with each other. This creates a strong bond and oxygen molecules are more stable in this form than individual atoms. Oxygen is typically found in this form in the atmosphere due to this extra stability.
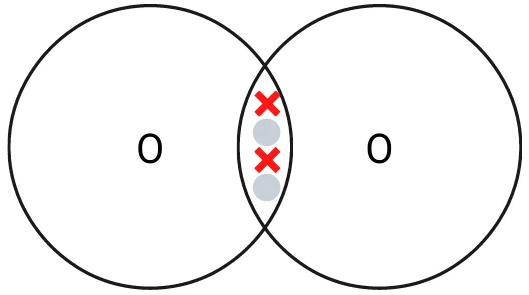
Covalent bonding of an oxygen molecule. Remember that many oxygen molecules are often present in the same area and weak intermolecular forces will hold them together.
How many bonds can oxygen form?
Oxygen can form 1 double bond with another oxygen atom. This is where two electrons from each oxygen atom are shared with another oxygen atom. In total, this means that 4 electrons are shared.
Check out the links below to try out GCSE chemistry quizzes or see all of our GCSE science quizzes.
