Mock test. 1 hour and 10 minutes. 60 marks available.
Scroll past the periodic table to start the test.
Mock test. 1 hour and 10 minutes. 60 marks available.
Scroll past the periodic table to start the test.
0 of 39 Questions completed
Questions:
|
You must fill out this field. |
|
|
You must fill out this field. |
|
|
You must fill out this field. |
|
|
You must fill out this field. |
You have already completed this quiz. You cannot start it again.
Quiz is loading…
You must sign in or sign up to take this quiz.
You must first complete the following:
Quiz complete. Results are being recorded.
0 of 39 Questions answered correctly
Your Time:
Time has elapsed.
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average Score |
|
| Your Score |
|
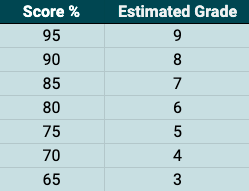
The above shows an estimate of your grade. Note: these grades are quite harsh and are under review. Taking part in this paper gives us more information to help us calibrate these grades.
Drag and drop to match the density with the state of matter
| Most dense state | |
| Least dense state | |
Match the change of state with the transition using the words below.
Freezing, sublimation, evaporation or boiling, condensation, melting, deposition
Drag and drop to match the description to the term
| Contains only a particular type of atoms | |
| Contains more than one type of atoms, chemically bonded together | |
| Contains more than one type of atoms, but not chemically bonded together | |
![]()
The graph shows a heating curve for substance X (red) and substance Y (blue). Drag and drop to match the substance to the correct description.
| Pure | |
| Impure | |
What is the chemical symbol for copper?
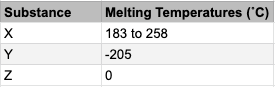
The table shows 3 substances and their melting points. What common substance, in particular, could substance Z be?
Which common, very important substance melts or freezes at 0 degrees C?
Drag and drop to match the charge with the correct particle
| +1 | |
| -1 | |
| 0 | |
Drag and drop to match the description to the correct number
| Number of protons | |
| Number of protons plus neutrons | |
True or false. All atoms of the same element have the same atomic number.
Which of the following things were hypothesised by John Dalton in 1805?
Atomic number represents the number of protons in the nucleus of an atom. This means that elements further down the table have:
In the modern periodic table, what are rows and columns called?
Mendeleev did not know about isotopes, this caused his periodic table to have pair reversals where elements with a higher atomic weight were placed before elements with a lower atomic weight. Which subatomic particles are found in different numbers in isotopes?
What is the electronic configuration of boron?
Filtration and crystallisation are used to separate which types of materials?
When a mixture of rocks, salt and water is filtered, what will be the residue?
Fractional distillation is used to separate which types of materials?
Use some of the following words to complete the statements below. You may use each word more than once.
Diamond, graphite, tetrahedral, delocalised, intermolecular, lattice, covalent, ionic
Complete the following statements:
How do sodium and chlorine atoms bond?
How many sulfur atoms will react with two lithium atoms to form an ionic compound?
What do group one atoms do when they bond ionically with group 7 atoms?
Which of these best describes metallic bonding?
Why is magnesium a better electrical conductor than sodium?
Halogens react with metals to form metal halides. Which kind of bond holds them together?
A soluble base is called?
What do acids produce when they dissolve in water?
Can strong and weak acids have the same pH?
Match the formula to the compound
Sodium hydroxide, hydrochloric acid, potassium hydroxide, nitric acid, calcium hydroxide, sulfuric acid
H₂SO₄ =
NaOH =
HCl =
KOH =
Ca(OH)₂ =
HNO₃ =
Solutions of less than pH 7 are?
Strong acids do what in water?
Calculate the empirical formula of a compound that contains 69.94g of Fe (Aᵣ – 55.85) and 30.06g of O (Aᵣ – 12.00).
Determine the empirical formula for Cyclopentane – C₅H₁₀?
Closed, non-enclosed, neutralisation, precipitation, equal, non-equal
Complete the statement describing a chemical reaction:
Potassium iodide solution and lead nitrate solution react in a closed flask to form a yellow precipitate of lead iodide and a colourless solution of potassium nitrate. This is a reaction and since it occurs in a closed flask it is an example of a system. The mass of the reactants and products will be .
Complete the following half equation for a reaction the cathode during electrolysis of molten zinc chloride. Zn²⁺ + ….. → Zn
Complete the following half equation for a reaction the cathode during electrolysis of molten lead bromide. Pb²⁺ + 2e → …..
During electrolysis which electrode do anions and cations migrate to?
Place the metals in the correct order – from highest to lowest reactivity
View Answers:
Which of the following is the correct description of the reactivity of metals?
Copper can be purified using electrolysis of copper sulfate solution. For this process, we use the setup in the image below. Complete the half equation for the reaction at the anode. Cu → ….. + …..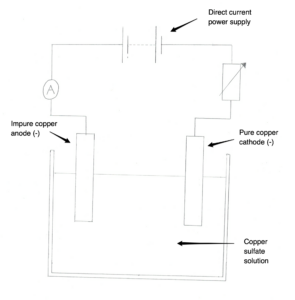
This paper covers the absolute basics of chemistry which are required to move on with the course. Students are tested on their understanding of states of matter which are fundamentally important to understanding the concepts of acids and alkalis. This first chemistry paper aims to test a student’s knowledge of topics that will help them to make sense of the world around them as well as understand the risks associated with certain materials. Additionally, the test will prepare them for A-level study or can be useful for a number of job roles by using the tools of logic in the workplace.
In this paper, the concepts of atomic structure which again are fundamental to later topics are addressed in CC1. CC2 continues on from CC1 with the periodic table and elements, along with concepts like electronic configurations and the basics of interactions between elements. CC5 tests students’ knowledge of ionic bonding which presupposes an understanding of the atomic structure and subatomic particles. CC6 adds in covalent bonding before CC7 addresses the rest of the bonding models which attempt to explain the properties of the materials around us. Acids and alkalis are next in CC8 where the students are expected to understand reactions between different materials, and the products and annotate or write balanced chemical equations including state symbols. Half and balanced ionic equations are assessed depending on the level. CC9 tests knowledge of masses, moles and empirical formulae. CC10 asks about electrolytic processes which link to CC11, obtaining and using metals and finally, CC12, reversible reactions and equilibria.
If you feel like you’re ready, move on to the next mock test – Edexcel Combined Science Paper 4: Chemistry 2.
© 2024 Discover Tutoring.
Please take a second to rate us on Google! We appreciate your help and support.
Click here to support us, it doesn’t cost anything and it only takes 1 minute.
If you appreciate our work and want to support us, please follow the below link and give us a rating on Google.
Click here to support us, it doesn’t cost anything and only takes 1 minute.
Better Luck Next Time!
You can check where you made any mistakes by closing this window and selecting “View Correct Answers”. Why not try again and see if you can improve on your score?
Great Work, You Passed the Quiz!
You can check where you made any mistakes by closing this window and selecting “View Correct Answers”.
Thanks for your review. Please help us improve by answering a few questions.
