In metallic bonding, electrostatic attraction between negative electrons and positive metal ions bonds the metal together. The lattice structure with free electrons allows metals to conduct electricity and heat whilst being strong and malleable.
Metallic Bonding Explained
How do Metals Conduct Electricity?
Metals are able to conduct electricity because when they bond, each atom donates its spare electron’s from its outer shell to a delocalised pool. Since electrons have a charge of -1, electrons in the delocalised pool are able to move and carry a charge. This also explains why metals are good conductors of heat since electrons can also move thermal energy.
When a voltage (potential difference) is applied, the delocalised electrons move in the same direction and an electrical current flows.
Metallic bonding also explains the differences in electrical conductivity between different metals. Metals which are in groups 1, 2 and 3 of the periodic table all have different electrical conductivities because they have contrasting numbers of electrons in their outermost shell. The group number tells us the number of electrons in the outer shell of an element and since all of these electrons are donated to a delocalised pool, metals with a higher group number such as aluminium have a higher conductivity than metals with a lower group number such as sodium.
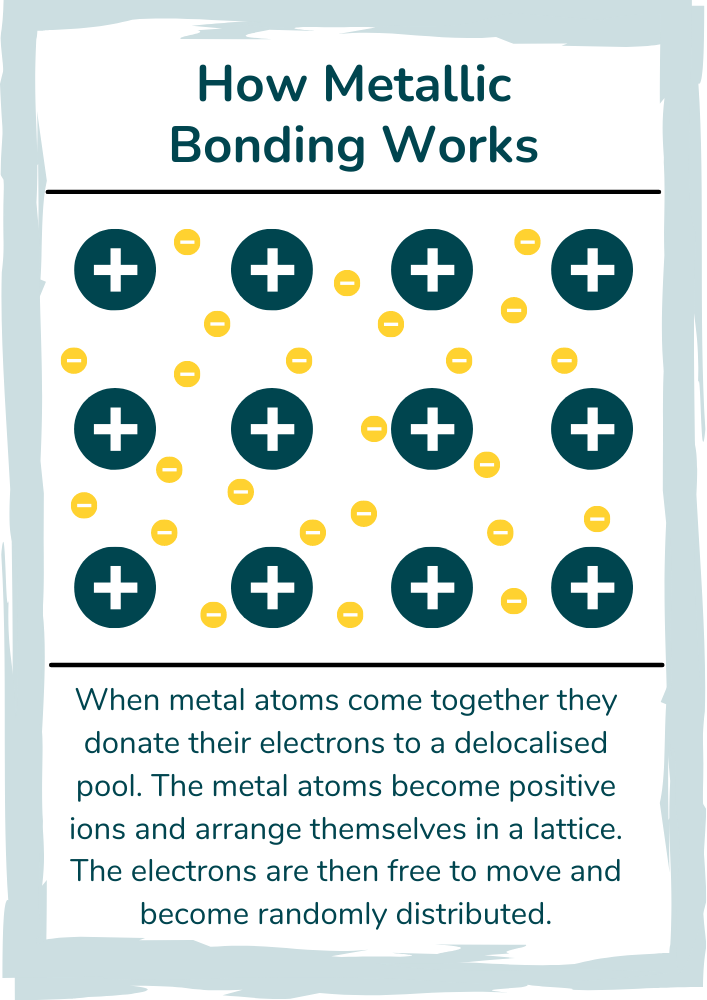
Metallic structure - The structure of metals
The diagram above shows the structure of metals (metallic structure). Metal ions automatically arrange themselves in a lattice structure which maximises the distance between each ion (since the nuclei are positively charged, they repel each other). The ions are held by a mutual electrostatic attraction to the delocalised pool of electrons.
What are delocalised electrons?
Delocalised electrons are electrons which have been lost or removed from their orbits around the nuclei of atoms. When electrons are stripped from the nucleus, the atom becomes an ion and gains a positive charge. The electrostatic attraction between the positive nucleus continues to attract the delocalised electrons after they leave, although the greater the distance, the weaker the force of attraction.
Why are Metals Malleable?
Metals are malleable because when they bond metallically, they form uniform layers with a delocalised pool of electrons holding the ions together. These layers are strongly attracted to the delocalised pool but not to each other, so it is possible for the layers to slide over each other, rather than crack or break.
In case you’re not sure, malleable means that metals can be bent and shaped without breaking, the opposite would be brittle like glass or pottery.
The electrostatic bonds between metal ions are very strong and this contributes to their hardness and also their high melting and boiling points. In order to break apart the bonds between the metal ions, lots of energy is required and therefore high temperatures or pressures.
Elements also bond through two other bonding models – covalent bonding and ionic bonding.
For more fundamental chemistry knowledge, check out the atomic structure quiz and the elements, compounds and mixtures quiz.
Try the Quizzes below to test your knowledge of bonding in chemistry!
