When mixed with water, acid and alkalis produce hydrogen and hydroxide ions, respectively. These chemicals are corrosive and should be kept away from the skin and clothes, through proper handling and safety procedures. The dangers of chemicals should be obvious from their hazard symbols.
Acids and Alkalis Explained
What are Bases?
Bases are substances that can neutralise acids, typically forming a salt. These materials are usually metal oxides or carbonates, such as magnesium oxide. Alkalis are bases that are soluble in water.
Bases are commonly used in cleaning materials like detergents but are also used in some industrial chemical processes and in manufacturing. Acidic solutions are also readily found around the home in substances such as vinegar, though some of the main uses are in industrial and chemical processes. For example, sulfuric acid is used in the manufacturing of fertilisers as well as in more mundane settings such as car batteries.
Formulas for acids and alkailis
The acids and alkalis formulas quiz aims to build your knowledge of the topic from the fundamentals onwards.
Remembering some basic formulas and examples of acids, alkalis and polyatomic ions will help you to understand the equations and make everything seem a bit less daunting. Polyatomic ions are ions that are made up of multiple atoms and they often appear in acids and alkalis. The most important ones to remember for GCSE are carbonate (CO32-), sulfate (SO42-), sulfite (SO32-), nitrate (NO3–), ammonium (NH4+) and hydroxide (OH–). These ions are especially useful to remember because they can explain what is happening in neutralisation reactions between acids and bases. Just take a look at the sulfate ion (SO42-) it is not a coincidence that sulfuric acid has the formula H2SO4. Sulfuric acid if formed by two positive hydrogen ions which have lost their electrons coming together with the sulfate ion.
Another example of why the polyatomic ions are useful to remember is the formulas for metal hydroxides which are bases. These have formulas that include the hydroxide ion. For instance, NaOH is the formula for sodium hydroxide and if you understand that it was formed when a sodium ion came together with a hydroxide ion, you can understand the following reaction:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Overall, remembering the formulas for the basic polyatomic ions as well as for some common acids and alkalis can really help you along.
What are neutralisation reactions?
Neutralisation reactions occur when acids and bases are combined, this produces predictable results when you understand the general formula. Additionally, it helps to understand what acids and bases are. Bases react to produce salts and water when they meet with acids. Metal oxides and hydroxides are bases and some are also alkalis which are a subsection of bases that dissolve in water. Sodium hydroxide for example is soluble and is a base that makes it an alkali. Acids are chemicals that contain hydrogen and release these hydrogen ions when they react with water. Hydrochloric acid contains hydrogen and chlorine, these dissociate when reacting with water as described by the following equation:
HCl (aq) → H⁺(aq) + Cl⁻(aq)
The above reaction demonstrates the action of acids breaking down into their components when in water. The state symbols (aq) after the hydrogen and chlorine ions show that they are dissolved in water. Bases can also be shown using these equations, just like the reaction between magnesium oxide and sulfuric acid to produce magnesium sulfate and water:
MgO (s) + H₂SO₄ (aq) → MgSO₄ (aq) + H₂O (l)
The name of the compound produced by acids reacting with metal oxides and hydroxides depends on the type of base and type of acid. The first part of the name is always the metal and then the second part comes from the acid. If the acid was sulfuric, the salt would be a sulfate. If the acid was hydrochloric, the salt would be chloride and so on.
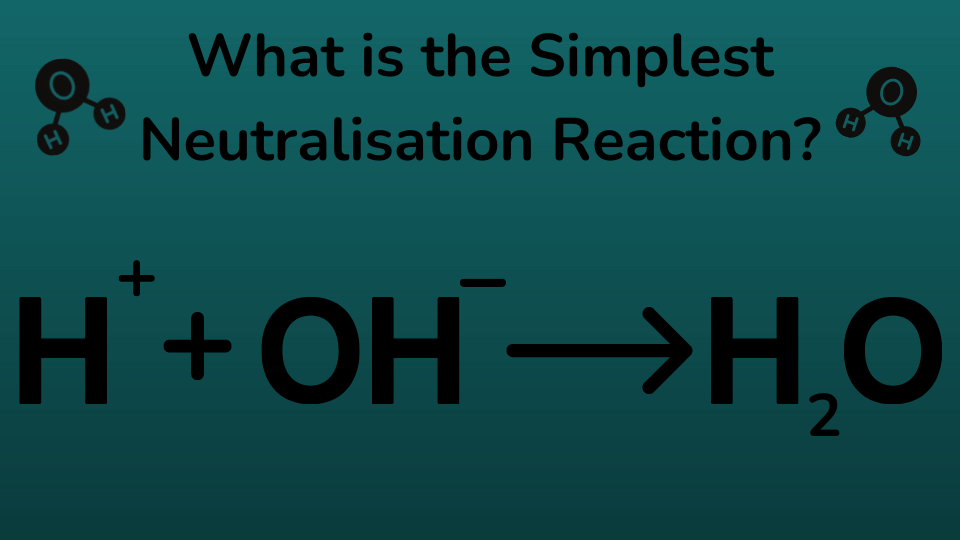
What name is given to a reaction in which hydroxide ions react with hydrogen ions to produce water?
This reaction is called a neutralisation reaction, it occurs when positive hydrogen ions react with hydroxide ions to produce water. Aqueous hydrogen ions (H⁺) are acidic and aqueous hydroxide ions (OH⁻) are basic. When mixed they become neutral or pH 7, making water (H₂O)