Do you know the difference between exothermic and endothormic?
Exothermic and Endothermic Reactions Quiz
Good Luck!
Is Photosynthesis Endothermic or Exothermic?
Photosynthesis is an endothermic reaction because it requires energy from the sun in order to form glucose.
Photosynthesis can also occur with artificial light sources and follows the same equation:
carbon dioxide + water → glucose + oxygen.
This reaction is often shown with sunlight (or light) above the arrow and chlorophyll below the arrow. This indicates that these are required for the reaction to take place but do not contribute to the products or reactants.
Find out more about reactions with our rates of reaction quiz.
Exothermic and Endothermic Reaction Examples
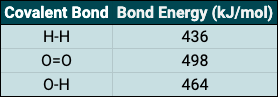
Endothermic reactions take in energy (usually in the form of thermal energy) from their surroundings, this leads to heating. Solutions can be good examples of this and as an example, if calcium chloride dissolves in water the temperature of the solution will increase. Exothermic reactions do the opposite, releasing energy into the environment. When ammonium chloride dissolves in water, the temperature of the solution will decrease, indicating an exothermic reaction has taken place.
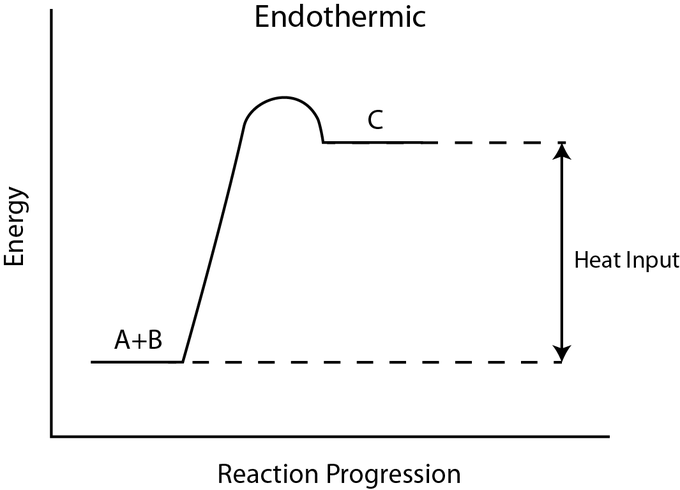
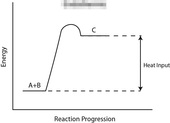
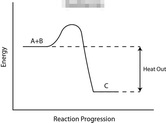
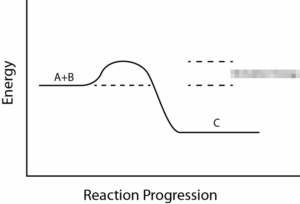
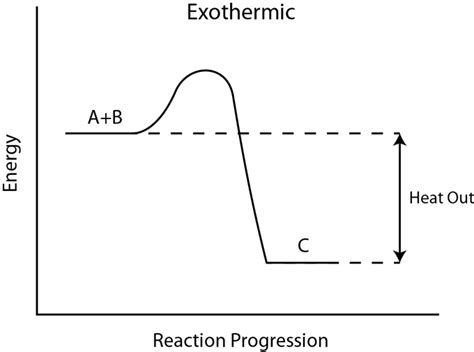
Take a look at the reaction profiles below to see how these energy changes take place.
Exothermic Reaction Profile
Endothermic Reaction Profile