Mock test. 1 hour and 15 minutes. 70 marks available.
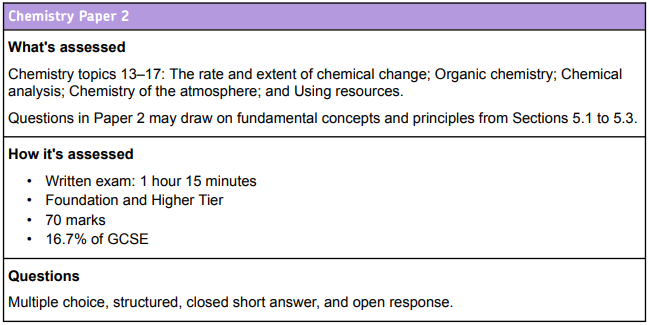
GCSE AQA Combined Science Trilogy Chemistry Paper 2
Good Luck!
Quiz Summary
0 of 54 Questions completed
Questions:
Information
You have already completed this quiz. You cannot start it again.
Quiz is loading…
You must sign in or sign up to take this quiz.
You must first complete the following:
Results
Quiz complete. Results are being recorded.
Results
0 of 54 Questions answered correctly
Your Time:
Time has elapsed.
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average Score |
|
| Your Score |
|
Categories
- Not categorized 0%
-

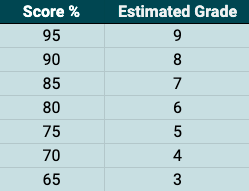
The above shows an estimate of your performance in this quiz. Note: these grades are quite harsh and are under review. Taking part in this paper gives us more information to help us evaluate these grades.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 54Question 11 Point
Which of these reasons explains why temperature affects rate of reaction?
CorrectIncorrect -
Question 2 of 54Question 21 Point
When there are more frequent collisions between particles, what will happen to the rate of reaction?
CorrectIncorrect -
Question 3 of 54Question 31 Point
Increasing the concentration of solutions increases the rate of reaction, why is this the case?
CorrectIncorrect -
Question 4 of 54Question 41 Point
Which of the following symbols is used to represent a reversible reaction?
CorrectIncorrect -
Question 5 of 54Question 53 Points
Which of the following are conditions that are used during the Haber process?
CorrectIncorrect -
Question 6 of 54Question 61 Point
The environment in which a reaction takes place can affect the direction of the equilibrium shift.
Hydrogen reacts with iodine to form hydrogen iodide. Match the change with the correct outcome.
H₂(g) + I₂(g) ⇌ 2HI(g)Fill in the blanks with the following terms:
Right, left
-
Match the following changes with the correct outcome:
- Increasing the concentration of H₂ will shift the equilibrium to the .
- Decreasing the pressure of I₂ will shift the equilibrium to the .
CorrectIncorrect -
-
Question 7 of 54Question 71 Point
The environment in which a reaction takes place can affect the direction of the equilibrium shift.
Hydrogen reacts with iodine to form hydrogen iodide. Match the change with the correct outcome.
H₂(g) + I₂(g) ⇌ 2HI(g)- Equilibrium shifts to the right
- Equilibrium shifts to the left
- H₂ is added
- H₂ is removed
CorrectIncorrect -
Question 8 of 54Question 81 Point
The environment in which a reaction takes place can affect the direction of the equilibrium shift.
A reaction is exothermic in the forwards direction. Match the change in temperature with the correct outcome.
- Equilibrium shifts to the left
- Equilibrium shifts to the right
- Increasing temperature
- Decreasing temperature
CorrectIncorrect -
Question 9 of 54Question 91 Point
There is a dynamic equilibrium formed in the following reaction: nitrogen dioxide ⇌ dinitrogen tetroxide. Which of the following will happen if we add more nitrogen dioxide?
CorrectIncorrect -
Question 10 of 54Question 103 Points
Use the following words to fill in the blanks:
Open, closed, active, dynamic, static
-
- equilibrium only occurs in systems.
- In an system gases can escape so equilibrium would not be achieved.
CorrectIncorrect -
-
Question 11 of 54Question 111 Point
The following equations show cracking of a long hydrocarbon into a shorter alkane and an alkene. Which equation is correct?
CorrectIncorrect -
Question 12 of 54Question 121 Point
What is the purpose of cracking?
CorrectIncorrect -
Question 13 of 54Question 131 Point
Which descriptions are accurate about saturated and unsaturated hydrocarbons?
CorrectIncorrect -
Question 14 of 54Question 141 Point
Which of the following is a difference between alkanes and alkenes?
CorrectIncorrect -
Question 15 of 54Question 151 Point
Crude oil separated by fractional distillation creates unequal volumes of each fraction e.g. fuel oil. These unequal volumes also do not match the demand for each fraction. What process is carried out to balance out the supply with demand?
CorrectIncorrect -
Question 16 of 54Question 161 Point
Alkanes all have the same general formula. What is that formula?
CorrectIncorrect -
Question 17 of 54Question 171 Point
Renewable, non-renewable
-
Complete the sentence: Methane is a fossil fuel found in natural gas.
CorrectIncorrect -
-
Question 18 of 54Question 181 Point
Hydrocarbons are compounds that contain which two elements only?
CorrectIncorrect -
Question 19 of 54Question 191 Point
Hydrocarbons with the longest carbon chains have the highest boiling points. Where are the fractions with the smallest carbon chains removed in a fractionating column?
CorrectIncorrect -
Question 20 of 54Question 201 Point
Which of the following statements are correct about crude oil?
CorrectIncorrect -
Question 21 of 54Question 211 Point
Which process do we use to separate crude oil into more useful and simpler mixtures?
CorrectIncorrect -
Question 22 of 54Question 221 Point
Why is bitumen not a good fuel?
CorrectIncorrect -
Question 23 of 54Question 231 Point
Renewable, non-renewable
-
Finish the sentence: Petrol, kerosene and diesel oil are fossil fuels obtained from crude oil.
CorrectIncorrect -
-
Question 24 of 54Question 241 Point
Which of the following explains dynamic equilibrium?
CorrectIncorrect -
Question 25 of 54Question 251 Point
A paper chromatography experiment is carried out in a lab. The distance moved by the spot was 6cm and the solvent had moved by 8cm. Calculate the Rf value.
CorrectIncorrect -
Question 26 of 54Question 261 Point
Can Rf values be higher than 1?
CorrectIncorrect -
Question 27 of 54Question 271 Point
The distance travelled by a spot is determined by the solubility of the compound. How does solubility impact distance travelled?
CorrectIncorrect -
Question 28 of 54Question 281 Point
What is the equation for Rf values in paper chromatography?
CorrectIncorrect -
Question 29 of 54Question 291 Point
Which of the following are uses for paper chromatography?
CorrectIncorrect -
Question 30 of 54Question 301 Point
Drag and drop to match the description to the phase of chromatography
- Mobile phase
- Stationary phase
- The solvent
- The paper
CorrectIncorrect -
Question 31 of 54Question 314 Points
Use some of the following words to fill in the blanks.
Earthquakes, volcanism, condensed, rivers, oceans, photosynthesis
-
- The development of the early atmosphere was mainly from which released gases from inside the Earth.
- The formation of the oceans happened when water vapour in the atmosphere as the Earth cooled.
- A decrease in CO₂ in the atmosphere occurred as CO₂ dissolved into .
- An increase in O₂ in the atmosphere occurred when organisms started the process of
CorrectIncorrect -
-
Question 32 of 54Question 321 Point
Which of the following are effects of climate change?
CorrectIncorrect -
Question 33 of 54Question 331 Point
Which steps can we take to reduce the impact of climate change on humanity or prevent further climate change?
CorrectIncorrect -
Question 34 of 54Question 341 Point
Which of the following is the chemical test for oxygen?
CorrectIncorrect -
Question 35 of 54Question 352 Points
Use some of the following words:
Magnetic, volcanic, magnetospheres, detritivore, volcanoes
-
On early Earth, there was a lot of activity as the planet was cooling down. We can link this to the formation of the atmosphere by measuring the gases released by and comparing them to the composition of the atmosphere over time
Correct 2 / 2 PointsIncorrect / 2 Points -
-
Question 36 of 54Question 361 Point
Why do historical measurements by humans of global temperature have some uncertainty?
CorrectIncorrect -
Question 37 of 54Question 371 Point
Why is carbon and carbon monoxide produced during incomplete combustion?
CorrectIncorrect -
Question 38 of 54Question 381 Point
Why does burning of fuel inside an internal combustion engine lead to the production of oxides of nitrogen?
CorrectIncorrect -
Question 39 of 54Question 391 Point
Which of the following are causes of acid rain?
CorrectIncorrect -
Question 40 of 54Question 401 Point
Which of the following are negative effects of acid rain?
CorrectIncorrect -
Question 41 of 54Question 411 Point
Which description best matches complete combustion?
CorrectIncorrect -
Question 42 of 54Question 422 Points
Which of the following points are advantages of recycling?
CorrectIncorrect -
Question 43 of 54Question 431 Point
Drag and drop to match the advantage and disadvantage of recycling compared to extraction from ores
- Mining ores damages the landscape
- Waste metals can be difficult to sort
- Advantage
- Disadvantage
CorrectIncorrect -
Question 44 of 54Question 441 Point
Consider the following hypothetical scenario:
- A company that makes electronic devices needs cobalt.
- The company is also responsible for the disposal of the devices at the end of their useful lives.
- A life-cycle assessment determines that it consumes 10,000 kilojoules per kg to produce cobalt through mining ore and 50,000 kilojoules to produce it from recycling.
- The energy required for disposal is 100,000 kilojoules per kg for mined cobalt and only 50,000 kilojoules per kg for recycled cobalt.
What should the company do?
CorrectIncorrect -
Question 45 of 54Question 454 Points
Use some of the following words to fill in the blanks.
Noise, landfill, river, habitats, dust
-
Complete the following statements about the advantages of recycling compared to the extraction of metals from ores.
- Mining produces and pollution
- Mining can damage important
- Less waste metal ends up in sites
CorrectIncorrect -
-
Question 46 of 54Question 461 Point
A life-cycle assessment determines that 100,000 kilojoules of energy will be consumed to produce 1kg of magnesium from an ore. Alternatively, it would consume 25,000 kilojoules to extract the same mass of magnesium through recycling. Which method should be used given this information?
CorrectIncorrect -
Question 47 of 54Question 471 Point
How could we accurately monitor the rate of a reaction?
CorrectIncorrect -
Question 48 of 54Question 481 Point
Drag and drop to match the outcome with the type of reaction
- Exothermic
- Endothermic
- If there is no energy input – reactions continue until reactants run out
- If there is no energy input – reactions stop even if there are reactants left
CorrectIncorrect -
Question 49 of 54Question 491 Point
Temperature affects the rate of reaction. Which of the following is the correct description of what happens when temperature changes?
CorrectIncorrect -
Question 50 of 54Question 501 Point
Surface area to volume ratio affects rate of reaction. Why is that the case?
CorrectIncorrect -
Question 51 of 54Question 512 Points
Ammonia is produced as a reversible reaction between which elements?
CorrectIncorrect -
Question 52 of 54Question 521 Point
Can the process used to obtain ammonia achieve dynamic equilibrium?
CorrectIncorrect -
Question 53 of 54Question 534 Points
Use some of the following words to fill in the blanks:
Hydrogen, nitrogen, oxygen, chlorine, air, natural, artificial, water
-
Complete the statements about the elements used in the process to make ammonia:
- is extracted from the .
- is obtained from gas
CorrectIncorrect -
-
Question 54 of 54Question 541 Point
Select the accurate statements about alkanes.
CorrectIncorrect
Is this higher or foundation?
This is a combined paper for higher and foundation students. You can achieve grades 1 – 9 on this paper, so it is suitable for all. Grades are moderated against the average result to give the most accurate indication of your performance. You can look at – How is this paper marked? for more information.
How is this paper marked?
This paper is automatically marked to determine which questions were answered correctly.
Your grade is determined using a Z-Score moderation system. Your GCSE exams are also moderated comparably so that the difficulty of papers is taken into account.
Roughly, this works by calculating your overall percentage and comparing it to the average percentage and the standard deviation. This means that for harder papers you need fewer points to get the same grade as you would for an easier paper.
As more students attempt the paper, the average score and standard deviation more accurately represent the difficulty of the paper and the grades become more accurate.
Making these papers and the marking system took considerable effort so if you found them helpful for your revision, please show your appreciation by rating the page.
Which exam board are you studying?