Atomic models are hypotheses and theories of atomic structure, including the subatomic particles and how they are arranged. These theories aim to describe the structure of atoms and explain their behaviour and characteristics.
Atomic Models Explained
The Rutherford Model
Models of atomic structure have developed over time as new discoveries of particles have given us more and more information.
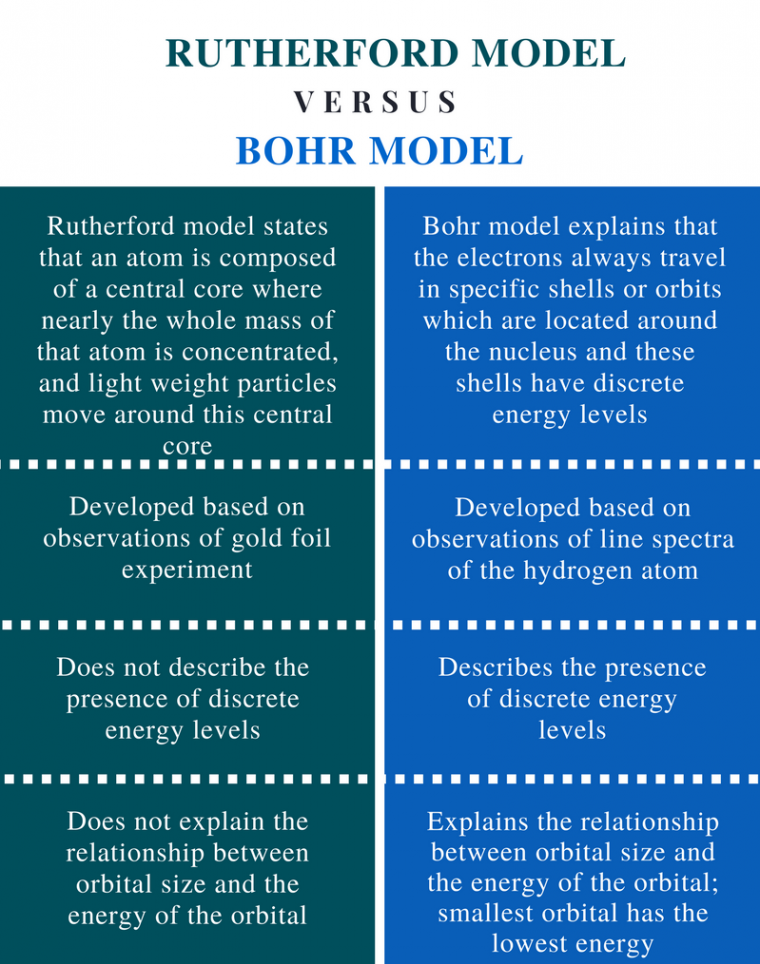
We currently use the Bohr Model to explain atomic structure, this is an extension of the Rutherford Model. The major difference between the Bohr and Rutherford Models is the explanation of why negatively charged electrons do not fall into the positively charged nucleus of atoms.
Bohr’s Model tells us that the electrons are arranged in specific energy levels and need to gain or lose energy to move to different levels. Building a more complete picture of the atom can help scientists to explain phenomena like radioactive decay and background radiation.
The table shows the differences between the Bohr and Rutherford Models.
The Rutherford Gold Foil Experiments
Ernest Rutherford and his team developed this theory through experiments with gold foil and alpha particles. They hypothesised that if the Plum Pudding Model was correct, the particles should all travel through undeflected.
What happened during these experiments?
- Alpha particles were fired at a thin sheet of gold foil.
- Most of the particles went straight through the foil.
- Some were deflected at large angles or even straight back.
What do the results tell us?
- Atoms are mostly empty space since most of the alpha particles travelled straight through the foil.
- Since only a few particles were deflected at large angles, it suggests that most of the positive material is concentrated towards the centre of atoms.
- The very small number of particles deflected back suggests that nuclei are tiny and direct collisions are very unlikely.
Do you remember all of the physics equations? Visit our GCSE Physics Equations page for all the equations you need plus calculators to check your answers! Also, check out our GCSE Electricity Equations page and calculators.
The Plum Pudding Model
JJ. Thomson proposed the Plum Pudding model after his groundbreaking discovery of negatively charged particles inside atoms. These particles are, of course, electrons.
The Plum Pudding Model suggests that atoms are masses of positively charged material with negatively charged particles dotted around. This arrangement resembles a plum pudding, also known as a Christmas Pudding.

JJ. Thomson is credited with discovering the electron after a series of experiments in 1897.
John Dalton's Atomic Model
John Dalton (1766 – 1844) was an English scientist of many disciplines. He focussed on understanding what the world was made of which led him to suggest that all matter is made of atoms and that they were indivisible fundamental particles. His theory had 4 main points:
- matter is made of atoms
- atoms are very small, hard and spherical
- atoms cannot be made and they are indestructible
- atoms in an element are identical
Not all of this was correct, but this was a useful theory to explain the properties of matter and a platform for other scientists to continue their work.
John Dalton was an early thinker on atomic structure. Building on the work of the ancient Greek philosopher Democritus (c 460 – 370 bc).
Now try the quizzes below to test your knowledge!