Do you know the units for activity?
Half-Life Quiz
Good Luck!
Quiz Summary
0 of 11 Questions completed
Questions:
Information
You have already completed this quiz. You cannot start it again.
Quiz is loading…
You must sign in or sign up to take this quiz.
You must first complete the following:
Results
Quiz complete. Results are being recorded.
Results
0 of 11 Questions answered correctly
Your Time:
Time has elapsed.
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average Score |
|
| Your Score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 11Question 1
Match the definition with the correct term. The …. of a substance is the number of nuclear decays per second.
CorrectIncorrect -
Question 2 of 11Question 2
Which of the following units do we use to measure the number of nuclear decays per second?
CorrectIncorrect -
Question 3 of 11Question 3
What is the term used to describe the amount of time taken for half of a sample to decay?
CorrectIncorrect -
Question 4 of 11Question 4
Which of the following statements is accurate?
CorrectIncorrect -
Question 5 of 11Question 5
Match the following definition with the correct term. …… is the time taken for the activity of a source to decay by half.
CorrectIncorrect -
Question 6 of 11Question 6
Which two of the following statements accurately describe the decay of unstable nuclei?
CorrectIncorrect -
Question 7 of 11Question 7
The half-life of a 25kg sample of caesium-137 is 30 years. What is the half life of a 12.5kg sample of caesium-137?
CorrectIncorrect -
Question 8 of 11Question 8
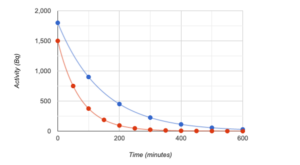
The graph shows the activity of two different radioactive substances. Determine the half-life of Source A (blue) in minutes. Do not add the units.
 CorrectIncorrect
CorrectIncorrect -
Question 9 of 11Question 9
The graph shows the activity of two different radioactive substances. Determine the half-life of Source B (red) in minutes. Do not add the units.
 CorrectIncorrect
CorrectIncorrect -
Question 10 of 11Question 10
The half-life of cobalt-60 is 5 years. If there are 100g of cobalt-60 in a sample, how much will be left after 15 years?
CorrectIncorrect -
Question 11 of 11Question 11
A sample of uranium-235 has a mass of 15kg. Given that the half-life of uranium-235 is 700 million years, calculate how long ago the sample would have been a mass of 240kg
CorrectIncorrect