Do you know where oxidation takes place?
Electrolysis Quiz
Quiz Summary
0 of 15 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 15
1. Question
What is an electrolyte?
CorrectIncorrect -
Question 2 of 15
2. Question
Use some of the following words to fill in the blanks:
Active, direct, indirect, electrical, magnetic
-
Fill in the blanks: Electrolysis is a process in which energy from a current supply is used to decompose electrolytes.
Correct 2 / 2 PointsIncorrect / 2 Points -
-
Question 3 of 15
3. Question
During electrolysis which electrode do anions and cations migrate to?
CorrectIncorrect -
Question 4 of 15
4. Question
Use some of the following words to fill in the blanks:
Gain, loss, anode, cathode-
Oxidation refers to the of electrons and it occurs at the
Correct 2 / 2 PointsIncorrect / 2 PointsHint
Use some of the following words:
cathode
anode
loss
gain
-
-
Question 5 of 15
5. Question
Complete the following half equation for a reaction the cathode during electrolysis of molten zinc chloride. Zn²⁺ + ….. → Zn
CorrectIncorrect -
Question 6 of 15
6. Question
Complete the following half equation for a reaction the cathode during electrolysis of molten lead bromide. Pb²⁺ + 2e → …..
CorrectIncorrect -
Question 7 of 15
7. Question
Which electrode would the ion Mg²⁺ migrate to during electrolysis?
CorrectIncorrect -
Question 8 of 15
8. Question
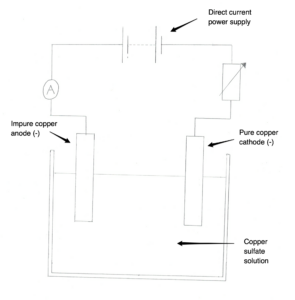
Copper can be purified using electrolysis of copper sulfate solution. For this process, we use the setup in the image below. Complete the half equation for the reaction at the anode. Cu → ….. + …..
 CorrectIncorrect
CorrectIncorrect -
Question 9 of 15
9. Question
Copper can be purified using electrolysis of copper sulfate solution. For this process, we use the setup in the image below. Complete the half equation for the reaction at the cathode. Cu²⁺ + 2e →
 CorrectIncorrect
CorrectIncorrect -
Question 10 of 15
10. Question
When using inert electrodes to perform electrolysis with copper chloride solution, which products will be produced at the anode and cathode?
CorrectIncorrect -
Question 11 of 15
11. Question
When using inert electrodes to perform electrolysis with sodium chloride solution, which products will be produced at the anode and cathode?
CorrectIncorrect -
Question 12 of 15
12. Question
[question number = 06.2] When using inert electrodes to perform electrolysis with sodium sulfate solution, which products will be produced at the anode and cathode?
CorrectIncorrect -
Question 13 of 15
13. Question
When using inert electrodes to perform electrolysis with water acidified with sulfuric acid, which products will be produced at the anode and cathode?
CorrectIncorrect -
Question 14 of 15
14. Question
[question number = 06.1] When using inert electrodes to perform electrolysis with molten lead bromide, which products will be produced at the anode and cathode?
CorrectIncorrect -
Question 15 of 15
15. Question
Use some of the following words to fill in the blanks:
Loss, gain, anode, cathode-
Fill in the blanks: Reduction refers to the of electrons and it occurs at the
Correct 2 / 2 PointsIncorrect / 2 PointsHint
Use some of the following words:
anode
cathode
loss
gain
-
Which exam board are you studying?