Mock test. 70 marks available.
GCSE AQA Combined Science Trilogy Chemistry Paper 2
Quiz Summary
0 of 32 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 32 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 6 Mark Questions 0%
- Short Answer 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 32
1. Question
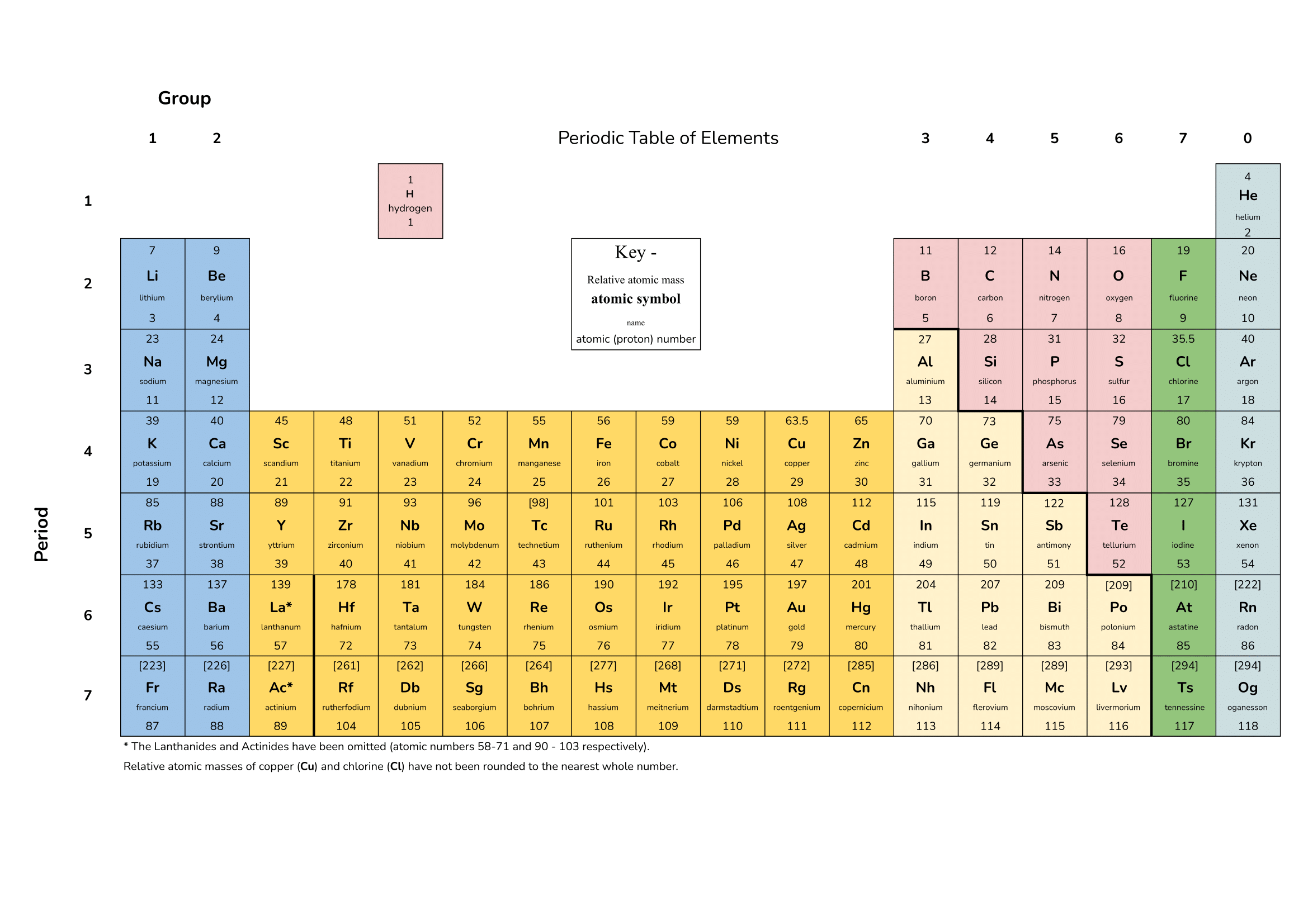
2 point(s)[question number = 01.1] Select the accurate statements about alkanes.
Correct 2 / 2 PointsIncorrect / 2 Points -
Question 2 of 32
2. Question
1 point(s)[question number = 01.2] Alkanes all have the same general formula. What is that formula?
CorrectIncorrect -
Question 3 of 32
3. Question
4 point(s)(short answer) [question number = 01.3] List the names of the first 4 alkanes in the series
Correct 4 / 4 PointsIncorrect / 4 Points -
Question 4 of 32
4. Question
1 point(s)[question number = 01.4] Which descriptions are accurate about saturated and unsaturated hydrocarbons?
CorrectIncorrect -
Question 5 of 32
5. Question
1 point(s)(short answer) [question number = 01.5] Crude oil separated by fractional distillation creates unequal volumes of each fraction e.g. fuel oil. These unequal volumes also do not match the demand for each fraction. What process is carried out to balance out the supply with demand?
CorrectIncorrect -
Question 6 of 32
6. Question
1 point(s)(short answer) [question number = 01.6] Alkanes and alkenes are both part of a series, what is the name we give to the series?
CorrectIncorrect -
Question 7 of 32
7. Question
1 point(s)[question number = 01.7] The following equations show cracking of a long hydrocarbon into a shorter alkane and an alkene. Which equation is correct?
CorrectIncorrect -
Question 8 of 32
8. Question
1 point(s)[question number = 01.8] Hydrocarbons are compounds that contain which two elements only?
CorrectIncorrect -
Question 9 of 32
9. Question
1 point(s)[question number = 01.9] Why is bitumen not a good fuel?
CorrectIncorrect -
Question 10 of 32
10. Question
1 point(s)(short answer) [question number = 02.1] Chemical reactions require a high enough collision energy for reactions to take place. What do we call the minimum amount of energy needed for a reaction?
CorrectIncorrect -
Question 11 of 32
11. Question
1 point(s)(short answer) [question number = 02.2] When there are more frequent collisions between particles, what will happen to the rate of reaction?
CorrectIncorrect -
Question 12 of 32
12. Question
6 point(s)[question number = 02.3] Explain in detail the different factors that can affect the rate of chemical reactions. Include explanations of how and why each factor influences the rate. (enter your answer)
Correct 6 / 6 PointsIncorrect / 6 Points -
Question 13 of 32
13. Question
6 point(s)[question number = 02.4] A student investigated the rate of gas production when different concentrations of sodium thiosulfate react with hydrochloric acid to produce sulfur dioxide.
Describe a method the student could use to measure the rate of reaction. (enter your answer)
You should include:
- how to set up the apparatus and the materials you would use
- the measurements you would make
- how you would control other variables
Correct 6 / 6 PointsIncorrect / 6 Points -
Question 14 of 32
14. Question
6 point(s)[question number = 02.5] (short answer) The hydrogenation of ethene is represented by the reversible reaction:
\(\mathrm{C_{2}H_{4(g)} + H_{2(g)} \rightleftharpoons C_{2}H_{6(g)}}\)
The bond energies of the bonds involved are shown in the table:
Bond Energy (kJ/mol) C–H 412 C=C 612 C–C 348 H–H 436 Predict and explain the effect of decreasing the temperature on the amount of Ethane produced.
Correct 6 / 6 PointsIncorrect / 6 Points -
Question 15 of 32
15. Question
1 point(s)[question number = 02.6] The environment in which a reaction takes place can affect the direction of the equilibrium shift.
Hydrogen reacts with iodine to form hydrogen iodide. Match the change with the correct outcome.
H₂(g) + I₂(g) ⇌ 2HI(g)Sort elements
- Equilibrium shifts to the right
- Equilibrium shifts to the left
- H₂ is added
- H₂ is removed
CorrectIncorrect -
Question 16 of 32
16. Question
1 point(s)[question number = 02.7] The environment in which a reaction takes place can affect the direction of the equilibrium shift.
A reaction is exothermic in the forwards direction. Match the change in temperature with the correct outcome.
Sort elements
- Equilibrium shifts to the left
- Equilibrium shifts to the right
- Increasing temperature
- Decreasing temperature
CorrectIncorrect -
Question 17 of 32
17. Question
3 point(s)[question number = 02.8] Use the following words to fill in the blanks:
Open, closed, active, dynamic, static
-
- equilibrium only occurs in systems.
- In an system gases can escape so equilibrium would not be achieved.
CorrectIncorrect -
-
Question 18 of 32
18. Question
1 point(s)[question number = 02.9] There is a dynamic equilibrium formed in the following reaction: nitrogen dioxide ⇌ dinitrogen tetroxide. Which of the following will happen if we add more nitrogen dioxide?
CorrectIncorrect -
Question 19 of 32
19. Question
1 point(s)[question number = 03.1] A paper chromatography experiment is carried out in a lab. The distance moved by the spot was 6cm and the solvent had moved by 8cm. Calculate the Rf value.
CorrectIncorrect -
Question 20 of 32
20. Question
1 point(s)[question number = 03.2] The distance travelled by a spot is determined by the solubility of the compound. How does solubility impact distance travelled?
CorrectIncorrect -
Question 21 of 32
21. Question
1 point(s)[question number = 03.3] What is the equation for Rf values in paper chromatography?
CorrectIncorrect -
Question 22 of 32
22. Question
1 point(s)[question number = 03.4] Which of the following are uses for paper chromatography?
CorrectIncorrect -
Question 23 of 32
23. Question
1 point(s)[question number = 03.5] Drag and drop to match the description to the phase of chromatography
Sort elements
- Mobile phase
- Stationary phase
- The solvent
- The paper
CorrectIncorrect -
Question 24 of 32
24. Question
4 point(s)[question number = 04.1] Which steps can we take to reduce the impact of climate change on humanity or prevent further climate change?
Correct 4 / 4 PointsIncorrect / 4 Points -
Question 25 of 32
25. Question
6 point(s)(short answer) [question number = 04.2] Describe the evolution of Earth’s atmosphere from the formation of the Earth 4.6 billion years ago to the atmosphere. This should not include any changes as a result of human activity (the increases in carbon dioxide following the industrial revolution).
Correct 6 / 6 PointsIncorrect / 6 Points -
Question 26 of 32
26. Question
2 point(s)[question number = 04.3] Why do historical measurements by humans of global temperature have some uncertainty?
Correct 2 / 2 PointsIncorrect / 2 Points -
Question 27 of 32
27. Question
1 point(s)[question number = 05.1] Which of the following is the chemical test for oxygen?
CorrectIncorrect -
Question 28 of 32
28. Question
1 point(s)[question number = 05.2] Why is carbon monoxide produced during incomplete combustion?
CorrectIncorrect -
Question 29 of 32
29. Question
2 point(s)[question number = 05.3] Which of the following are causes of acid rain?
Correct 2 / 2 PointsIncorrect / 2 Points -
Question 30 of 32
30. Question
2 point(s)[question number = 06.1] Which of the following points are advantages of recycling?
Correct 2 / 2 PointsIncorrect / 2 Points -
Question 31 of 32
31. Question
4 point(s)[question number = 06.2] (short answer) Describe two processes that can be used to extract metals from low-grade ores.
Correct 4 / 4 PointsIncorrect / 4 Points -
Question 32 of 32
32. Question
4 point(s)[question number = 06.3] Use some of the following words to fill in the blanks.
Noise, landfill, river, habitats, dust
-
Complete the following statements about the advantages of recycling compared to the extraction of metals from ores.
- Mining produces and pollution
- Mining can damage important
- Less waste metal ends up in sites
CorrectIncorrect -
Is this higher or foundation?
This is a combined paper for higher and foundation students. You can achieve grades 1 – 9 on this paper, so it is suitable for all. Grades are moderated against the average result to give the most accurate indication of your performance. You can look at – How is this paper marked? for more information.
How is this paper marked?
This paper is automatically marked to determine which questions were answered correctly.
Your grade is determined using a Z-Score moderation system. Your GCSE exams are also moderated comparably so that the difficulty of papers is taken into account.
Roughly, this works by calculating your overall percentage and comparing it to the average percentage and the standard deviation. This means that for harder papers you need fewer points to get the same grade as you would for an easier paper.
As more students attempt the paper, the average score and standard deviation more accurately represent the difficulty of the paper and the grades become more accurate.
Making these papers and the marking system took considerable effort so if you found them helpful for your revision, please show your appreciation by rating the page.
Related Quizzes
Which exam board are you studying?