Mock test. 100 marks available.
GCSE AQA Chemistry Paper 1
Good Luck!
Quiz Summary
0 of 34 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 34 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 6 Mark Questions 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 34
1. Question
3 point(s)[question number = 01.1] Match the relative mass with the correct particle. Use some of the following numbers.
1, 2, 0, 1/1836
-
Protons =
Neutrons =
Electrons =
CorrectIncorrect -
-
Question 2 of 34
2. Question
3 point(s)[question number = 01.2] Drag and drop to match the charge with the correct particle
Sort elements
- Protons
- Electrons
- Neutrons
- +1
- -1
- 0
CorrectIncorrect -
Question 3 of 34
3. Question
5 point(s)[question number = 01.3] (short answer) Lithium, sodium and potassium are all Group 1 metals. When small pieces of these metals are added to water, different observations are made. Explain why the reactions become more vigorous as you go down Group 1
Correct 5 / 5 PointsIncorrect / 5 Points -
Question 4 of 34
4. Question
6 point(s)[question number = 01.4] Use some of the following words to complete the statements below. You may use each word more than once.
Increase, increases, decrease, decreases, lower, higher
-
Complete the following statements about the trends in the properties of noble gases as you go down the group:
- Melting points . e.g. helium has a melting point than neon
- Boiling points . e.g. neon has a melting point than argon
- Density . e.g. krypton has a density than argon
CorrectIncorrect -
-
Question 5 of 34
5. Question
3 point(s)[question number = 01.5] Drag and drop to match the description to the term
Sort elements
- Element
- Compound
- Mixture
- Contains only a particular type of atoms
- Contains more than one type of atoms, chemically bonded together
- Contains more than one type of atoms, but not chemically bonded together
CorrectIncorrect -
Question 6 of 34
6. Question
3 point(s)[question number = 02.1] Drag and drop to match the description and state of matter. Particles are:
Sort elements
- Liquids
- Solids
- Gases
- Close together and move around each other
- Close together and vibrate around fixed positions
- Far apart and moving quickly in random directions
CorrectIncorrect -
Question 7 of 34
7. Question
1 point(s)[question number = 02.2] What are the melting and boiling points of water?
Sort elements
- Melting point
- Boiling point
- Neither
- 0˚C
- 100˚C
- 50˚C
CorrectIncorrectHint
Drag and drop
-
Question 8 of 34
8. Question
1 point(s)[question number = 02.3] The melting point of sodium is 97.8˚C and the boiling point is 883˚C. What state is sodium at 120˚C?
CorrectIncorrect -
Question 9 of 34
9. Question
3 point(s)[question number = 03.1] (short answer) Two elements with the electronic structures 2,8,2 and 2,8,7 react together to form a compound. Name the compound formed, the type of compound formed and write a balanced equation for the reaction.
Correct 3 / 3 PointsIncorrect / 3 Points -
Question 10 of 34
10. Question
1 point(s)[question number = 03.2] What is the formula for the compound calcium nitrate which is made of calcium and nitrate ions (NO₃⁻)?
CorrectIncorrect -
Question 11 of 34
11. Question
1 point(s)[question number = 03.3] Since metallically bonded atoms have a delocalised pool of electrons, these electrons are able to conduct ……
Correct 1 / 1 PointsIncorrect / 1 Points -
Question 12 of 34
12. Question
1 point(s)[question number = 03.4] What is the name of the force that holds together ions in ionic compounds?
CorrectIncorrect -
Question 13 of 34
13. Question
6 point(s)(short answer) [question number = 03.5] Explain why graphite is often used as a lubricant while diamond is used in cutting tools. Link your answer to the structure and bonding in both substances.
Correct 6 / 6 PointsIncorrect / 6 Points -
Question 14 of 34
14. Question
2 point(s)[question number = 03.6] Fill in the blanks using some of the following answers:
Atoms, molecules, surface area, volume, nanometre, micrometre-
Nanoscience refers to structures that are 1–100 in size, of the order of a few hundred .
CorrectIncorrect -
-
Question 15 of 34
15. Question
6 point(s)[question number = 04.1] A student wants to investigate the order of reactivity of three metals (e.g., magnesium, zinc, and copper). They are not sure about the method that should be used.
Plan an investigation to determine the order of reactivity. (enter your answer)
You should include:
- How to set up the apparatus and the materials you would use
- How to collect and record data
- How to ensure accuracy and reliability of results
Correct 6 / 6 PointsIncorrect / 6 Points -
Question 16 of 34
16. Question
3 point(s)[question number = 04.2] An experiment was conducted to measure the rate of reaction of different metals with acids. The data shows how much gas was produced in 10 seconds in cm³. Use the data to determine the order of reactivity from highest to lowest.
Iron – 4.4
Magnesium – 12.9
Lead – 1.7
Tin – 2.3
Calcium – 15.0
Aluminium – 7.1
- Aluminium
- Tin
- Magnesium
- Lead
- Iron
- Calcium
View Answers:
CorrectIncorrect -
-
Question 17 of 34
17. Question
6 point(s)[question number = 04.3] (short answer) A student is investigating three unlabelled solutions: A, B, and C. The student uses universal indicator to test each solution and records these observations:
- Solution A turns the indicator red
- Solution B turns the indicator purple
- Solution C turns the indicator green
Explain what these results tell you about the solutions and describe what would happen if Solution A were mixed with Solution B. Include a balanced equation in your answer.
Tip – after typing a number press Control to convert to subscript, then again to superscript or use the buttons in the top right corner of the text area.Correct 6 / 6 PointsIncorrect / 6 Points -
Question 18 of 34
18. Question
4 point(s)(short answer) [question number = 04.4] A student has added calcium carbonate to dilute hydrochloric acid in a beaker until in excess. Describe the remaining steps in preparing a pure, dry sample of a soluble salt, including details of heating methods and equipment needed at each stage.
Correct 4 / 4 PointsIncorrect / 4 Points -
Question 19 of 34
19. Question
3 point(s)[question number = 05.1] Calculate the number of molecules in 16g of oxygen O₂.
CorrectIncorrect -
Question 20 of 34
20. Question
3 point(s)[question number = 05.2] You have a sample of 788g of gold which contains 4 moles of particles. Calculate the Aᵣ of gold.
CorrectIncorrect -
Question 21 of 34
21. Question
3 point(s)[question number = 05.3] Calculate the mass of oxygen needed to react with 20g of hydrogen to make water.
The balanced chemical equation for this reaction is:
2H₂ + O₂ → 2H₂O
-
g
CorrectIncorrect -
-
Question 22 of 34
22. Question
2 point(s)[question number = 05.4] Fill in the blanks to show the relationship between the different units of measurement for volume.
-
1dm³ = litre = cm³
CorrectIncorrect -
-
Question 23 of 34
23. Question
3 point(s)[question number = 05.5] Calculate the atom economy for the production of ethanol in the fermentation of glucose:
C₆H₁₂O₆ → 2C₂H₅OH + 2CO₂
Relative formula masses: C₆H₁₂O₆=180, C₂H₅OH=46, CO₂=44
Give your answer to 1 decimal place.
-
The atom economy is .
CorrectIncorrect -
-
Question 24 of 34
24. Question
3 point(s)[question number = 05.6]
Calculate the concentration in g/dm³ of a hydrochloric acid solution if 50.0 cm³ reacts completely with 25.0 cm³ of 0.100 mol/dm³ sodium hydroxide.
-
Concentration of HCl = g/dm³
CorrectIncorrect -
-
Question 25 of 34
25. Question
1 point(s)[question number = 06.1] When using inert electrodes to perform electrolysis with molten lead bromide, which products will be produced at the anode and cathode?
CorrectIncorrect -
Question 26 of 34
26. Question
1 point(s)[question number = 06.2] When using inert electrodes to perform electrolysis with sodium sulfate solution, which products will be produced at the anode and cathode?
CorrectIncorrect -
Question 27 of 34
27. Question
6 point(s)[question number = 06.3] Use some of the following words to fill in the blanks. You can use each word more than once.
Copper, carbon, hydrogen, oxygen, less, more, electrolysis, heating
-
Complete the statements:
- Metals that are reactive than are extracted through .
- Metals that are reactive than are extracted by in a furnace.
CorrectIncorrect -
-
Question 28 of 34
28. Question
3 point(s)[question number = 07.1] (short answer) Methane is commonly used in households for heating water in boilers for central heating. When methane burns completely, water and carbon dioxide are produced. Give the balanced chemical equation for this reaction and state whether it is exothermic or endothermic.
Tip – after typing a number press Control to convert to subscript, then again to superscript or use the buttons in the top right corner of the text area.
Correct 3 / 3 PointsIncorrect / 3 Points -
Question 29 of 34
29. Question
3 point(s)[question number = 07.2] Given your answer to question 7.1 and the table below. Calculate the bond energies involved in the reaction.
Bond Bond Energy (kJ/mol) C=C 612 C-H 412 C-O 358 O=O 498 -
- Bonds Broken (Reactants): kJ/mol
- Bonds Formed (Products): kJ/mol
- Net Energy Change: kJ/mol
CorrectIncorrect -
-
Question 30 of 34
30. Question
1 point(s)[question number = 07.3] Drag and drop to match the reaction to the correct type
Sort elements
- Endothermic
- Exothermic
- Takes in energy, making the surroundings cooler
- Transfers energy to the surroundings
CorrectIncorrect -
Question 31 of 34
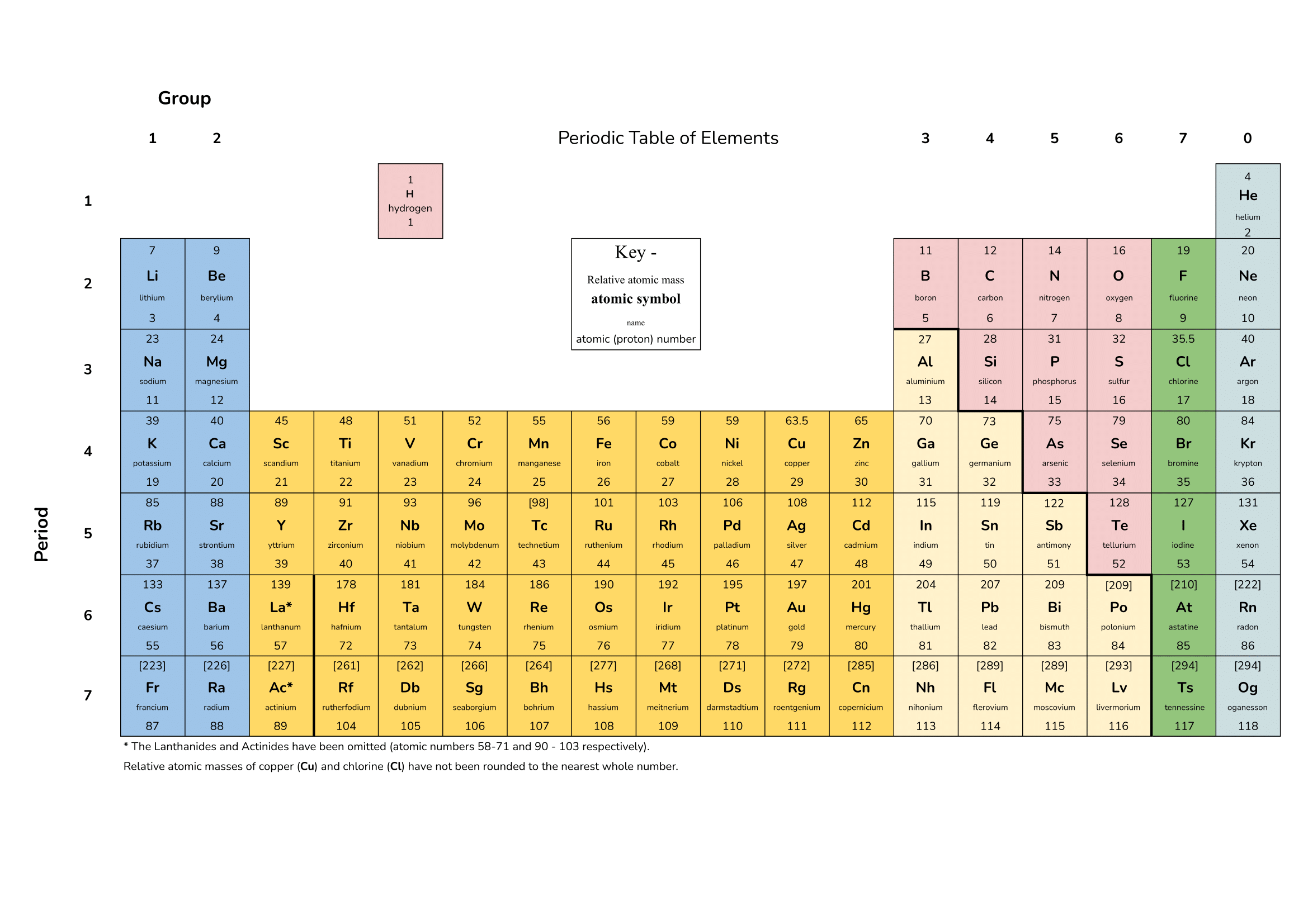
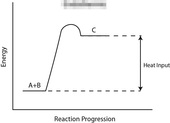
31. Question
1 point(s)[question number = 07.4] Reaction profiles are used to show the energy transfers during reactions. Drag and drop to match the reaction profile to the correct type of energy transfer.
Sort elements
- Endothermic
- Exothermic
CorrectIncorrect -
Question 32 of 34
32. Question
4 point(s)[question number = 08.1] Use some of the following answers to fill in the blanks:
Chemical, fuel, electrical, hydrogen, carbon-
Fuel cells use as a and produce an current through a reaction.
CorrectIncorrect -
-
Question 33 of 34
33. Question
3 point(s)(short answer) [question number = 08.2] In a hydrogen fuel cell, hydrogen gas reacts with oxygen gas to produce water. Write the overall equation for this reaction and the half equations occurring at each electrode in a hydrogen fuel cell.
Correct 3 / 3 PointsIncorrect / 3 Points -
Question 34 of 34
34. Question
2 point(s)[question number = 08.3] Use some of the following answers to fill in the blanks:
Electricity, electrolyte, voltage, electrode, metals, cells-
The voltage of a cell depends on factors like the type of and used in the cell.
CorrectIncorrect -
Is this higher or foundation?
This is a combined paper for higher and foundation students. You can achieve grades 1 – 9 on this paper, so it is suitable for all. Grades are moderated against the average result to give the most accurate indication of your performance. You can look at – How is this paper marked? for more information.
How is this paper marked?
This paper is automatically marked to determine which questions were answered correctly.
Your grade is determined using a Z-Score moderation system. Your GCSE exams are also moderated comparably so that the difficulty of papers is taken into account.
Roughly, this works by calculating your overall percentage and comparing it to the average percentage and the standard deviation. This means that for harder papers you need fewer points to get the same grade as you would for an easier paper.
As more students attempt the paper, the average score and standard deviation more accurately represent the difficulty of the paper and the grades become more accurate.
Making these papers and the marking system took considerable effort so if you found them helpful for your revision, please show your appreciation by rating the page.
Related Quizzes
Which exam board are you studying?